Research and development is the lifeblood of the bio/pharmaceutical industry. While lifecycle strategies and novel delivery systems can lessen revenue declines in the wake of patent expirations, drug companies can survive for only so long on reformulating older products. The industry must be able to introduce new therapies to maintain its growth and profitability. The pressure on bio/pharmaceutical companies’ R&D operations has never been greater. Companies need successful new products to rekindle growth and stem the loss of revenues and jobs following patent expirations. Because new clinical science is producing products that are more targeted than the broad market blockbusters they are replacing, R&D laboratories must produce a larger number of new products than ever before.
 R&D decline
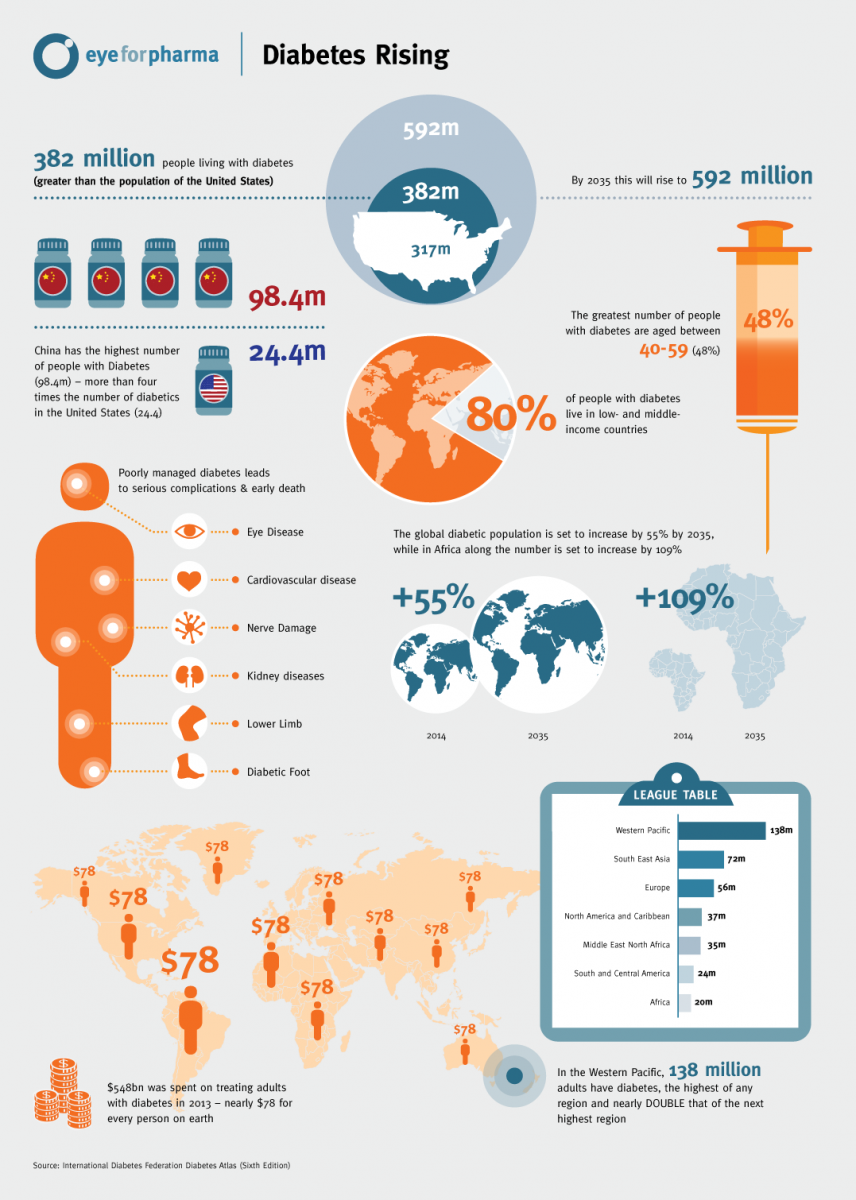
R&D decline Despite the imperative to accelerate new drug development, indications are that the number of compounds entering clinical development has actually been declining. The number of new investigation new drug (IND) filings at FDA has been declining since 2008, down by nearly one-third. Further, registrations of new Phase II and Phase III clinical trials in the US government’s clinicaltrials.gov database have dropped 15%, with new filings by global bio/pharmaceutical companies down by more than 20%.
What is behind the apparent decline in R&D activity? It seems to be the confluence of a number of events that has upset R&D productivity, such as:
Global bio/pharmaceutical companies are redesigning their R&D model by narrowing their therapeutic focus, laying off scientists, dismantling isolated research campuses in favor of research facilities in medical research hubs such as Boston and San Francisco, and in-licensing or acquiring more pipeline candidates from early-stage companies.
Bio/pharmaceutical research is establishing a new scientific foundation based on genomics and biologics. Genomics is still a maturing science while most global bio/pharmaceutical companies are still building their expertise and infrastructure for developing biopharmaceuticals.
Bio/pharmaceutical companies are killing candidates earlier in the pipeline, before they incur the high costs of clinical development. The practice reflects, in part, the greater understanding of what makes compounds toxic and better tools for assessing toxicity in early development. It also reflects the fact that insurers and governments are insisting that new drugs deliver greater efficacy than the products already available. Payers are no longer willing to pay for expensive therapies that provide just a few more weeks of life to a cancer patient or a marginal decline in LDL (low-density lipoprotein) levels, for example, as an anticholesterol drug. Only drugs that deliver substantial clinical benefits are being advanced through the development pipeline.
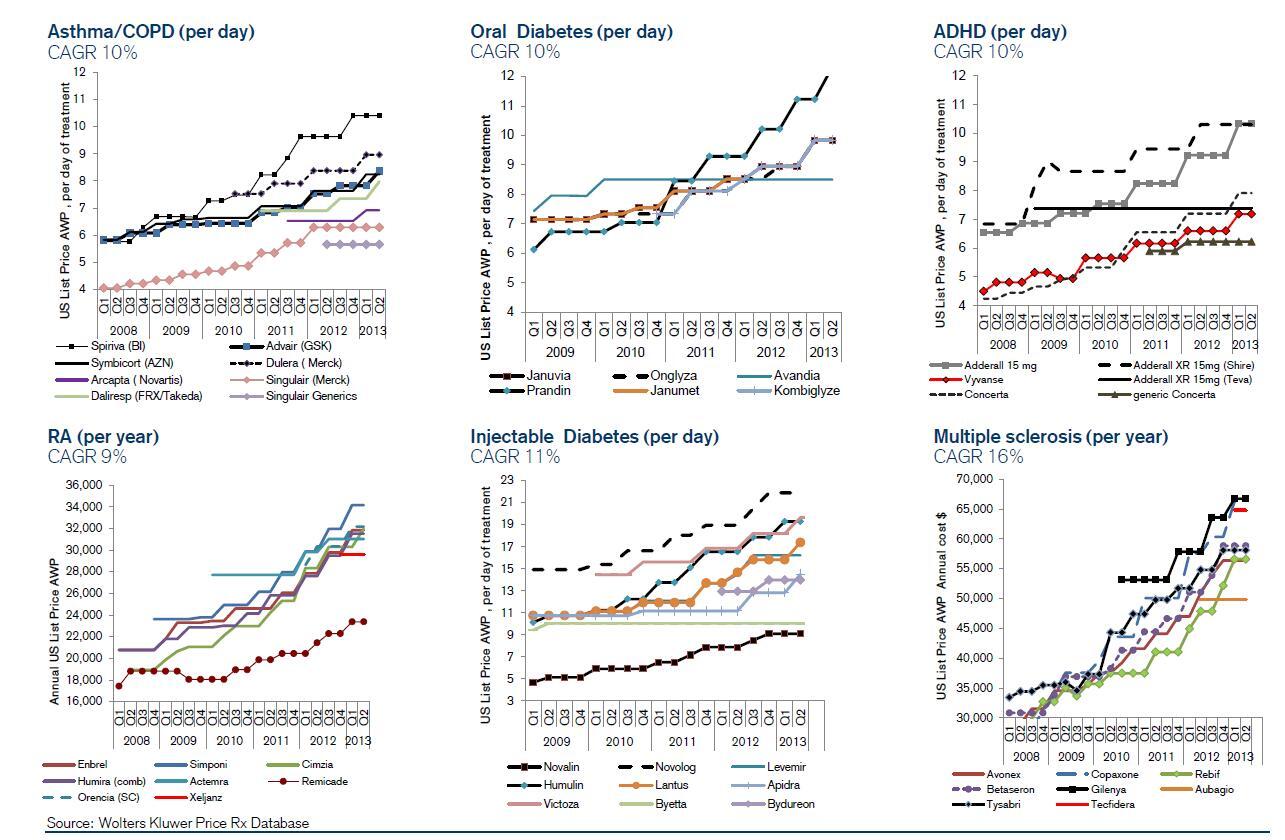
Late-stage clinical development is getting more expensive. Bio/pharmaceutical companies are saving money by delaying expensive formulation, methods development, and toxicology testing until after proof-of-concept is demonstrated. However, other factors are driving up the cost of clinical development. The need to prove therapeutic superiority means clinical studies must be conducted against marketed products, which must be purchased and blinded at great expense, rather than placebos. FDA guidance often requires more extensive compound characterization and analytical testing. Enrolling enough patients to get statistically significant results has become increasingly difficult and expensive.
Smaller companies have been afraid to spend money too rapidly for fear that they won’t be able to replace spent funds, which has limited willingness to spend.
Whether the decline in R&D activity is permanent or transitory is a crucial question for the entire bio/pharmaceutical industry, and especially for CROs and CDMOs that support research and development. If R&D doesn’t rebound, the growth prospects of R&D services providers will suffer, and even commercial CMOs will see fewer opportunities coming their way.
The future of R&D
It is likely that most of the negative trends impacting R&D activity will correct themselves in due course. The disruption in R&D programs caused by the elimination of therapeutic programs and closure of R&D operations will settle down. The Silicon Valley-like cross-fertilization of ideas that bio/pharmaceutical companies are hoping to instigate by establishing R&D operations in medical research hubs will start to bear fruit. And financing for high-risk R&D is likely to return as the re-opening of the initial public offering window will convince venture capitalists that profitable exits are still possible.
Two factors, however, will continue to create strong headwinds for the growth of R&D: payers will continue to demand demonstrable efficacy and cost advantages for new drugs and the cost of R&D to demonstrate therapeutic value and cost-effectiveness will continue to escalate.
Those new demands will create new opportunities and challenges for CDMOs and CROs. The opportunities come from bio/pharmaceutical companies seeking to control R&D costs by turning to contract service providers, the costs of which can be made variable, over in-house capabilities whose costs are fixed. Benefits will also accrue to CDMOs and CROs that can come up with innovative, cost-effective solutions to the problem of controlling R&D costs.
The challenges will arise from the pressures on prices and margins as bio/pharmaceutical companies seek to negotiate better terms to manage their costs. They will also come from the continuous churn in projects as R&D programs are cancelled when they don’t meet the raised performance hurdles.
CDMOs and CROs that expect a continuation of the “good old days” of the mid-2000s are not facing the realities of today’s bio/pharmaceutical research and development environment. The world has changed forever, and service providers need to face up to the new realities.