
Thursday, October 31, 2013
Targeted failure of the week. Post No 113. MM-121
Another mAbs messssss...
From here.
 Merrimack Pharmaceuticals Inc. (NASDAQ:MACK) fell $0.64 (19%) to $2.74 on Wednesday after reporting that MM-121 plus paclitaxel missed the primary endpoint of improving progression-free survival (PFS) vs. paclitaxel alone in a Phase II trial to treat platinum-resistant or refractory advanced ovarian cancer. In August, Merrimack said a pre-specified interim analysis showed MM-121 would not meet the endpoint. At the time, the company said it would continue the trial based on "expectations of the potential for subgroup benefit." MM-121 is a human mAb against epidermal growth factor receptor 3 (EGFR3; HER3; ErbB3).
Merrimack Pharmaceuticals Inc. (NASDAQ:MACK) fell $0.64 (19%) to $2.74 on Wednesday after reporting that MM-121 plus paclitaxel missed the primary endpoint of improving progression-free survival (PFS) vs. paclitaxel alone in a Phase II trial to treat platinum-resistant or refractory advanced ovarian cancer. In August, Merrimack said a pre-specified interim analysis showed MM-121 would not meet the endpoint. At the time, the company said it would continue the trial based on "expectations of the potential for subgroup benefit." MM-121 is a human mAb against epidermal growth factor receptor 3 (EGFR3; HER3; ErbB3).
From here.
 Merrimack Pharmaceuticals Inc. (NASDAQ:MACK) fell $0.64 (19%) to $2.74 on Wednesday after reporting that MM-121 plus paclitaxel missed the primary endpoint of improving progression-free survival (PFS) vs. paclitaxel alone in a Phase II trial to treat platinum-resistant or refractory advanced ovarian cancer. In August, Merrimack said a pre-specified interim analysis showed MM-121 would not meet the endpoint. At the time, the company said it would continue the trial based on "expectations of the potential for subgroup benefit." MM-121 is a human mAb against epidermal growth factor receptor 3 (EGFR3; HER3; ErbB3).
Merrimack Pharmaceuticals Inc. (NASDAQ:MACK) fell $0.64 (19%) to $2.74 on Wednesday after reporting that MM-121 plus paclitaxel missed the primary endpoint of improving progression-free survival (PFS) vs. paclitaxel alone in a Phase II trial to treat platinum-resistant or refractory advanced ovarian cancer. In August, Merrimack said a pre-specified interim analysis showed MM-121 would not meet the endpoint. At the time, the company said it would continue the trial based on "expectations of the potential for subgroup benefit." MM-121 is a human mAb against epidermal growth factor receptor 3 (EGFR3; HER3; ErbB3).
On Wednesday, Merrimack said an ongoing analysis of an undisclosed pre-specified set of biomarkers mechanistically linked to EGFR3 signaling identified a potential subpopulation of patients benefiting from MM-121 plus paclitaxel. Merrimack also said an increase in the rate of pulmonary embolism was reported in the MM-121 plus paclitaxel arm compared to the paclitaxel alone arm (5% vs. 1.2%). The company said that none of the pulmonary embolisms were fatal, led to treatment discontinuation or were assessed as treatment-related by the study investigators.
The open-label, international trial enrolled 223 patients with platinum-resistant or refractory advanced ovarian cancer. Merrimack said it will work with partner Sanofi (Euronext:SAN; NYSE:SNY) to determine next steps, but declined to disclose details.
Labels:
Big Pharma,
bubble,
Death Valley,
deconstruction,
failure,
Partisan of Death Valley,
targeted failure
Wednesday, October 30, 2013
Cancer - Big Pharma want to drain you of cash before you die (Health as a critical resource)
Labels:
Big Pharma,
bubble,
deconstruction,
evidence based medicine,
numbers,
paradigm,
politics,
real science
Tuesday, October 29, 2013
Monday, October 28, 2013
Another magic cure: Pervitin macht frei!
Labels:
another magic cure,
Big Pharma,
bubble,
cognitive,
deconstruction,
evidence based medicine,
real science,
video
Sunday, October 27, 2013
Saturday, October 26, 2013
Friday, October 25, 2013
Evolution of medicine

Labels:
bubble,
chaos,
cognitive,
deconstruction,
failure,
future foresight,
masterpiece of the day,
nice idea,
paradigm,
real science
Thursday, October 24, 2013
Be smart with insider trading!
Labels:
Big Pharma,
chaos,
cognitive,
deconstruction,
masterpiece of the day,
real science
Wednesday, October 23, 2013
Targeted drugs - the mantra!
Labels:
another magic cure,
Big Pharma,
bubble,
deconstruction,
nice idea,
paradigm,
real science,
targeted failure,
video
Caffeine suppositories? Why?
Labels:
another magic cure,
cognitive,
deconstruction,
failure,
shit of the day
Tuesday, October 22, 2013
Do you love Lenin as I do?
Big Pharma and fake?
 From here
From here
A Bristol-Myers Squibb researcher faked or falsified data in grant applications to the US National Institutes of Health and American Heart Association, as well as his PhD thesis and two published papers, according to the US Department of Health & Human Services Office of Research Integrity (see this). The disclosure was first reported by Retraction Watch.
Instances of falsified data have caused considerable embarrassment to other large drugmakers this year. Both Novartis (NVS) and GlaxoSmithKline (GSK) were embroiled in scandals in Japan and China, respectively (read here and here). However, the examples cited by the ORI apparently took place before the researcher began work at Bristol-Myers. A Bristol-Myers (BMY) spokeswoman declined to comment.
Labels:
Big Pharma,
bubble,
chaos,
cognitive,
deconstruction,
failure,
shit of the day
Monday, October 21, 2013
The 3 Best-Selling Drugs in the World
 The 3 Best-Selling Drugs in the World are here:
The 3 Best-Selling Drugs in the World are here:Humira -- estimated 2013 sales: $10.1 billion
Remicade -- estimated 2013 sales: $8.7 billion
Advair -- estimated 2013 sales: $8.6 billion
Just be impressed!
Labels:
another magic cure,
Big Pharma,
bubble,
evidence based medicine,
masterpiece of the day,
nice idea,
numbers,
real science
Sunday, October 20, 2013
The zests

Labels:
chaos,
cognitive,
deconstruction,
masterpiece of the day,
nice idea,
politics,
quantum,
real science,
semantic
Parallel reality.
Labels:
cognitive,
deconstruction,
politics,
real science,
semantic
Saturday, October 19, 2013
Targeted failure of the week. Post No 112. Ponatinib, Iclusig
 Ariad Pharmaceuticals Inc. (NASDAQ:ARIA) fell $1.83 (41%) to $2.67 on Friday after discontinuing the confirmatory Phase III EPIC trial evaluating Iclusig ponatinib in newly diagnosed leukemia patients due to arterial thrombotic events observed in patients treated with the drug. Ariad said the decision was based on an analysis of safety data from the trial that was conducted after FDA placed a partial clinical hold last week on all trials of Iclusig. EPIC had enrolled 307 patients of a planned 500.
Ariad Pharmaceuticals Inc. (NASDAQ:ARIA) fell $1.83 (41%) to $2.67 on Friday after discontinuing the confirmatory Phase III EPIC trial evaluating Iclusig ponatinib in newly diagnosed leukemia patients due to arterial thrombotic events observed in patients treated with the drug. Ariad said the decision was based on an analysis of safety data from the trial that was conducted after FDA placed a partial clinical hold last week on all trials of Iclusig. EPIC had enrolled 307 patients of a planned 500. Friday, October 18, 2013
3 days... Fake?

Labels:
cognitive,
masterpiece of the day,
paradigm,
Partisan of Death Valley,
politics,
real science
The 5 Diseases Americans Fear the Most or Health as a critical resource
 From here
From hereNo. 5: Diabetes (6%)
No. 4: Stroke (8%)
No. 3: Heart disease (8%)
No. 2: Alzheimer's disease (31%)
No. 1: Cancer (41%)
Labels:
Big Pharma,
bubble,
cognitive,
deconstruction,
future foresight,
real science
Thursday, October 17, 2013
Wednesday, October 16, 2013
Russian TV Delusion. And Ukraine will be free!
Labels:
chaos,
cognitive,
deconstruction,
failure,
future foresigh,
masterpiece of the day,
paradigm,
politics,
real science,
shit of the day,
targeted failure,
video
Targeted failure of the week. Post No 111. Krystexxa's pegylated disaster
Krystexxxxxxxxxxxa-hhhhkhkhkh. Well, it is not only targeted failure but a pegylated disaster:

And what happend?
Savient listed total assets of about $74 million and liabilities of $260 million as of June 30, court documents filed on Monday showed.

From Wiki: Pegloticase (or Krystexxa) is a tetrameric peptide composed of four identical chains of about 300 amino acids each. Approximately nine of the 30 lysine residues in each chain are pegylated. These side chains consist of about 225 ethylene glycol units each.
And what happend?
Savient listed total assets of about $74 million and liabilities of $260 million as of June 30, court documents filed on Monday showed.
Labels:
bubble,
Death Valley,
deconstruction,
depression,
failure,
real science,
shit of the day,
targeted failure
Tuesday, October 15, 2013
Monday, October 14, 2013
Quotes of the day



Labels:
another magic cure,
chaos,
cognitive,
deconstruction,
nice idea,
quote of the day,
real science
Sunday, October 13, 2013
Just tax planning
Labels:
cognitive,
deconstruction,
evidence based medicine,
future foresight,
nice idea,
numbers,
paradigm,
politics,
real science,
shit of the day,
video
Saturday, October 12, 2013
Targeted failure of the week. Post No 109 and 110. Vintafolide and stem cell business.
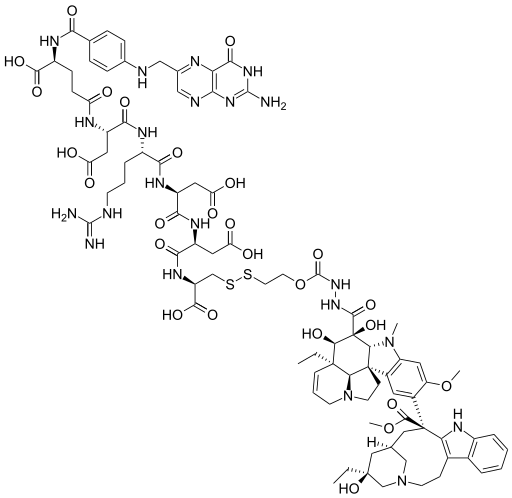 The idea with vintafolide is extremely sexy: From wiki: Vintafolide is a derivative of the anti-mitotic chemotherapy drug vinblastine[1] in which it is chemically linked to folic acid. Vintafolide was designed to deliver the toxic vinblastine group using folate targeting to cancer cells that overexpress the folic acid receptor.[2] Vintafolide is being studied for treatment of late-stage ovarian cancer and mid-stage non-small cell lung cancer.
The idea with vintafolide is extremely sexy: From wiki: Vintafolide is a derivative of the anti-mitotic chemotherapy drug vinblastine[1] in which it is chemically linked to folic acid. Vintafolide was designed to deliver the toxic vinblastine group using folate targeting to cancer cells that overexpress the folic acid receptor.[2] Vintafolide is being studied for treatment of late-stage ovarian cancer and mid-stage non-small cell lung cancer.
And guess what? Failure... Why? The sexy theory is not enough for the drug to be effective or, in other words, the theory is not ontologically correct.
See also here.
The same s..t is with stem cell business (and I guess it is the beginning ot the bubble burst): Osiris exits stem cell business, sells unit to Mesoblast in $100M deal
Labels:
Big Pharma,
bubble,
cognitive,
deconstruction,
evidence based medicine,
failure,
future foresight,
paradigm,
Partisan of Death Valley,
real science,
shit of the day,
targeted failure
Energy Mint as a citical resource?
 The post-modern society is not funny any more: The step-daughter of a man who died from a massive caffeine overdose after eating a pack of mints bought from his local sweet shop today demanded that they are immediately removed from sale.
The post-modern society is not funny any more: The step-daughter of a man who died from a massive caffeine overdose after eating a pack of mints bought from his local sweet shop today demanded that they are immediately removed from sale.
Labels:
Big Pharma,
bubble,
cognitive,
deconstruction,
failure,
future foresight,
paradigm,
politics,
real science,
shit of the day
Friday, October 11, 2013
Who was Mr Jobs?
Labels:
chaos,
cognitive,
deconstruction,
failure,
masterpiece of the day,
real science,
shit of the day
Thursday, October 10, 2013
Fake?

Labels:
chaos,
cognitive,
deconstruction,
depression,
masterpiece of the day,
nice idea,
paradigm,
politics,
quote of the day,
real science,
shit of the day
Targeted failure of the week. Post 108. Iclusig
Ariad Pharmaceuticals Inc said the U.S. Food and Drug Administration had placed a partial hold on patient enrollment for trials of its cancer drug Iclusig after a number of patients taking the drug experienced blood clots and heart damage.
Ariad shares plunged as much as 77 percent to $4.00 in heavy trading -- their lowest in three years.
The company said it paused enrollment in the trials after it reviewed new data from a pivotal mid-stage trial testing the drug to treat two forms of blood cancer.
Ariad shares plunged as much as 77 percent to $4.00 in heavy trading -- their lowest in three years.
The company said it paused enrollment in the trials after it reviewed new data from a pivotal mid-stage trial testing the drug to treat two forms of blood cancer.
Labels:
Big Pharma,
deconstruction,
failure,
targeted failure
Wednesday, October 9, 2013
LoL
Labels:
another magic cure,
bubble,
cognitive,
deconstruction,
failure,
future foresight,
investment,
nice idea,
paradigm,
real science,
video
Tuesday, October 8, 2013
Antibiotics: How we will win the war against bacteria
A new scientific mantra:
https://www.youtube.com/watch?v=IJoJTY4nj5g
https://www.youtube.com/watch?v=IJoJTY4nj5g
Labels:
another magic cure,
cognitive,
Death Valley,
deconstruction,
future foresight,
nice idea,
paradigm,
real science,
targeted failure,
video
Monday, October 7, 2013
Sunday, October 6, 2013
Honest Titanic
Labels:
another magic cure,
chaos,
cognitive,
deconstruction,
nice idea,
real science,
video
Music of the week.
Labels:
cognitive,
music of the week,
nice idea,
real science
Saturday, October 5, 2013
Friday, October 4, 2013
Top Biotechs Set to Win in the War on Cancer - cancer is scared to death!
 Tuesday saw the wrap up of the 2013 European Society of Medical Oncology (ESMO) conference in Amsterdam, which featured key data from several of the companies covered by the biotech analysts at UBS. While the UBS team came away from ESMO with a much clearer picture of the competitive positioning and clinical profiles for many of the drugs from companies under their coverage, on balance this year’s ESMO meeting was much less stock-moving event than in years past.
Tuesday saw the wrap up of the 2013 European Society of Medical Oncology (ESMO) conference in Amsterdam, which featured key data from several of the companies covered by the biotech analysts at UBS. While the UBS team came away from ESMO with a much clearer picture of the competitive positioning and clinical profiles for many of the drugs from companies under their coverage, on balance this year’s ESMO meeting was much less stock-moving event than in years past.
One thing from the conference was extremely evident. The biotech sector remains catalyst-rich for the remainder of 2013, with key data readouts at several upcoming conferences to impact shares. In a new research report, UBS highlights the top names in the biotechnology world making huge strides to bring an end to a disease that killed almost 600,000 people in the United States alone in 2012.
Fecal Transplant, Now in Pill Form. Shit of the day.
Do not try it at home? Gel caps containing concentrated fecal microbes stopped recurrent Clostridium difficile infection and were well-tolerated by recipients, researchers reported here.

Labels:
another magic cure,
chaos,
cognitive,
deconstruction,
depression,
future foresight,
paradigm,
real science,
shit of the day
Erection as a critical resource

 Call it the anti-Viagra. It's not a product for the man who can't get the party started. It's for the man who ends the party too soon.
Call it the anti-Viagra. It's not a product for the man who can't get the party started. It's for the man who ends the party too soon.Competition is heating up to treat premature ejaculation, or PE, which may afflict one-in-three men at some point in their lives—and not just when they're teenagers.
Promescent, an FDA-approved, over-the-counter spray to treat PE. The spray uses lidocaine to reduce sensitivity and allow for longer performance. It is absorbed quickly so as not to be transferred to sexual partners. From here: http://www.nbcnews.com/business/new-sex-drug-men-too-much-hurry-8C11329732
Thursday, October 3, 2013
The many - the better?
Three parents and a disease-free baby
The upside of having three genetic parents
The U.K. government will allow scientists to create babies using DNA from three different parents—a positive development, according to some researchers, for parents with mitochondrial diseases.
The controversial procedure—also called mitochondrial replacement—is possible through in vitro fertilization (IVF). Mitochondrial diseases like muscular dystrophy affect about one in 5,000 people worldwide, so only a very small group of people would be interested in such a procedure, Virginia Hughes writes in Popular Science. The procedure would "be performed at a few select clinics in the U.K. and will be carefully monitored...[a]nd if not safe, it will most likely be banned."
Labels:
chaos,
cognitive,
deconstruction,
depression,
future foresight,
politics,
shit of the day
Wednesday, October 2, 2013
Top 15 highest paid biopharma R&D chiefs
Tuesday, October 1, 2013
Quantum macromechanics?
Labels:
another magic cure,
chaos,
cognitive,
deconstruction,
magic,
nice idea,
paradigm,
quantum,
real science,
video
The Monty Hall Problem - Absolute mindf*ck!
Labels:
another magic cure,
cognitive,
deconstruction,
evidence based medicine,
magic,
masterpiece of the day,
nice idea,
numbers,
politics,
quantum,
real science,
video
Subscribe to:
Posts (Atom)



-N-Methamphetamine_structural_formulae.png)


















