Thursday, January 31, 2013
Healthy dose of propaganda: Goldman Sachs does not suck!
Labels:
bubble,
chaos,
deconstruction,
depression,
failure,
future foresight,
macro economy,
nice idea,
paradigm,
politics,
real science,
shit of the day,
video
Bioinformatics: the ultimate truth
I always knew that Bioinformatics is absolutely BS (as well as genomics, proteomics and relevant HTS tricks). And here I found the similar reasoning.
Bioinformatics is an attempt to make molecular biology relevant to reality. All the molecular biologists, devoid of skills beyond those of a laboratory technician, cried out for the mathematicians and programmers to magically extract science from their mountain of shitty results.
 And so the programmers descended and built giant databases where huge numbers of shitty results could be searched quickly. They wrote algorithms to organize shitty results into trees and make pretty graphs of them, and the molecular biologists carefully avoided telling the programmers the actual quality of the results. When it became obvious to everyone involved that a class of results was worthless, such as microarray data, there was a rush of handwaving about “not really quantitative, but we can draw qualitative conclusions” followed by a hasty switch to a new technique that had not yet been proved worthless.
And so the programmers descended and built giant databases where huge numbers of shitty results could be searched quickly. They wrote algorithms to organize shitty results into trees and make pretty graphs of them, and the molecular biologists carefully avoided telling the programmers the actual quality of the results. When it became obvious to everyone involved that a class of results was worthless, such as microarray data, there was a rush of handwaving about “not really quantitative, but we can draw qualitative conclusions” followed by a hasty switch to a new technique that had not yet been proved worthless.
And the databases grew, and everyone annotated their data by searching the databases, then submitted in turn. No one seems to have pointed out that this makes your database a reflection of your database, not a reflection of reality. Pull out an annotation in GenBank today and it’s not very long odds that it’s completely wrong.
Labels:
Big Pharma,
bubble,
chaos,
deconstruction,
depression,
evidence based medicine,
failure,
future foresight,
HTS,
nice idea,
paradigm,
Partisan of Death Valley,
real science,
shit of the day,
targeted failure
Shit of the day. WTF? It is not a voyage!!!
Here is the original (everything else is BS):
Labels:
another magic cure,
cognitive,
deconstruction,
failure,
real science,
semantic,
shit of the day,
video
Wednesday, January 30, 2013
Shit of the day: Nanomedicine and its experts
One ”expert” from here
tells some bogus to the readers regarding ”nanomedicine” like
that:
Do not work with positively charged
particles, they are toxic! WTF??? Stop reading this BS!!!
Labels:
bubble,
cognitive,
deconstruction,
depression,
failure,
nanotechnology,
paradigm,
shit of the day
Heidegger. The life of the genius
Labels:
another magic cure,
cognitive,
deconstruction,
magic,
paradigm,
politics,
quantum,
real science,
semantic,
video
Tuesday, January 29, 2013
Another magic cure against cancer. Post No 37. Targeted thorium conjugate
There are no chances to fail! It works as a real magic bullet! We have to get cancer cure! :)
 Immunomedics Inc. (NASDAQ:IMMU) granted Algeta ASA (OSE:ALGETA) rights to develop a targeted thorium conjugate (TTC) comprised of Algeta's thorium-227, an alpha-particle emitting radionuclide, linked to Immunomedics' epratuzumab for cancer. Immunomedics will provide Algeta with epratuzumab, and Algeta will fund all preclinical and clinical development through Phase I testing. After Phase I testing, Algeta has an option to license rights to the compound under undisclosed terms. Algeta will pay Immunomedics an undisclosed fee and an undisclosed antibody delivery milestone, plus payments for cGMP manufacturing. The partners declined to disclose additional details. Algeta was up NOK2.40 to NOK164.80 on Monday, while Immunomedics was up $0.08 to $2.81.
Immunomedics Inc. (NASDAQ:IMMU) granted Algeta ASA (OSE:ALGETA) rights to develop a targeted thorium conjugate (TTC) comprised of Algeta's thorium-227, an alpha-particle emitting radionuclide, linked to Immunomedics' epratuzumab for cancer. Immunomedics will provide Algeta with epratuzumab, and Algeta will fund all preclinical and clinical development through Phase I testing. After Phase I testing, Algeta has an option to license rights to the compound under undisclosed terms. Algeta will pay Immunomedics an undisclosed fee and an undisclosed antibody delivery milestone, plus payments for cGMP manufacturing. The partners declined to disclose additional details. Algeta was up NOK2.40 to NOK164.80 on Monday, while Immunomedics was up $0.08 to $2.81.
Labels:
another magic cure,
Big Pharma,
bubble,
deconstruction,
evidence based medicine,
failure,
future foresight,
nice idea,
Partisan of Death Valley,
shit of the day,
targeted failure
Targeted failure of the week. Post No 46. Naptumomab
It is basically not a surprise that mabs fail. A fresh failure is here:
 Active Biotech AB (SSE:ACTI) fell SEK7.50 (13%) to SEK51.50 on Monday after it said Anyara naptumomab estafenatox plus interferon (IFN) alpha missed the primary endpoint of improving overall survival (OS) vs. IFN alone in a European Phase II/III trial to treat renal cell carcinoma (RCC). Active Biotech said that an unexpected majority of the 513 patients had high levels of pre-formed antibodies against the superantigen component of Anyara, a 5T4 antibody combined with a mutated staphylococcal enterotoxin A (SEA). In a subgroup analysis of the 25% of patients with low or normal levels of baseline IL-6 and expected anti-superantigen antibody levels, Active Biotech said Anyara plus IFN alpha significantly improved OS (p=0.02) and the secondary endpoint of progression-free survival (p-value not disclosed) vs. IFN alpha alone.
Active Biotech AB (SSE:ACTI) fell SEK7.50 (13%) to SEK51.50 on Monday after it said Anyara naptumomab estafenatox plus interferon (IFN) alpha missed the primary endpoint of improving overall survival (OS) vs. IFN alone in a European Phase II/III trial to treat renal cell carcinoma (RCC). Active Biotech said that an unexpected majority of the 513 patients had high levels of pre-formed antibodies against the superantigen component of Anyara, a 5T4 antibody combined with a mutated staphylococcal enterotoxin A (SEA). In a subgroup analysis of the 25% of patients with low or normal levels of baseline IL-6 and expected anti-superantigen antibody levels, Active Biotech said Anyara plus IFN alpha significantly improved OS (p=0.02) and the secondary endpoint of progression-free survival (p-value not disclosed) vs. IFN alpha alone.
Labels:
Big Pharma,
bubble,
deconstruction,
depression,
failure,
paradigm,
shit of the day,
targeted failure
Monday, January 28, 2013
Pereslegin. The best of.
Labels:
another magic cure,
chaos,
cognitive,
Death Valley,
deconstruction,
future foresight,
magic,
nice idea,
politics,
quantum,
real science,
video
Russian roads.
Labels:
chaos,
deconstruction,
nice idea,
paradigm,
politics,
shit of the day,
video
Quote of the day. Soros.
Labels:
another magic cure,
bubble,
cognitive,
deconstruction,
future foresight,
investment,
macro economy,
nice idea,
paradigm,
politics,
real science
Sunday, January 27, 2013
'Let banks go bankrupt' - why not?
Only positive consequences!
Labels:
another magic cure,
bubble,
cognitive,
deconstruction,
failure,
future foresight,
macro economy,
paradigm,
politics,
real science,
video
'Using Indians as guinea pigs' - the ethics of Big Pharma
Video is here: http://www.smh.com.au/world/drug-companies-using-indians-as-guinea-pigs-20130126-2dduh.html#ixzz2J9s6FgtM
INDIA's Supreme Court has condemned the global pharmaceutical industry, claiming it has been using its citizens as ''guinea pigs''.
''It pains us that illiterate people and the children of India are being used as guinea pigs by the multinational drug companies … Uncontrolled clinical trials are creating havoc in the country,'' Justice R.M. Lodha said from the bench this month.
The court has imposed direct control of clinical trials on the health secretary, stripping authority from government agency the Drug Controller-General, whose inaction, the court said, was allowing multinational pharmaceutical companies to run ''rackets'' across India.
''You have to protect the health of the citizens of the country. It is your obligation. Deaths must be arrested and illegal trials must be stayed,'' the bench said.
Precise figures are hard to know, but health organisations estimate between 350,000 and 2 million Indians have been involved in the trials, some of which have been conducted using Australian government funding.
As revealed in a Fairfax Media investigation, clinical drug trials are at the centre of a growing controversy in India as evidence emerges before courts and in government inquiries of patients being put onto drug trials without their knowledge or consent, of patients dying and their families being left uncompensated, and of doctors being paid generous commissions to enlist as many subjects for trials as they can.
Doctors are being told what to say - word for word - by the drug manufacturers in their assessment of the drugs they are supposed to be trialling, a parliamentary committee has found.
And patients' rights groups such as Swasthya Adhikar Manch have uncovered hundreds of hospital records showing drug trials being conducted on patients without their permission, by, or on behalf of, some of the biggest names in the global pharmaceutical trade.
There is little deterrence: a dozen doctors found to have conducted secret drug trials on children and patients with learning difficulties were fined less than $100. It is a health system in crisis, observers say, harming those it is supposed to help.
Labels:
Big Pharma,
chaos,
deconstruction,
depression,
failure,
future foresight,
paradigm,
shit of the day,
video
Quote of the day. The majority fails...
Brandon Smith of Alt-Market blog writes:
If there is one concept on Earth that has been the absolute bane of human existence, it would have to be the concept of the “majority opinion”. The moment men began refusing to develop their own world views without first asking “What does everyone else think?”, they set themselves up for an endless future of failures. We are, of course, very social beings, and our natures drive us to seek those of like mind and spirit in what some might call a “tribal imperative”. Human beings desperately want to belong, but, they also desperately want to understand the environment around them. Often, the desire to belong and the desire to know the truth conflict. In some societies, in order to be accepted, one must give up on his search for truth and avoid eliciting the anger of others. This causes a severe mental and emotional disturbance within a population. In order to reconcile their conflicting needs within a system that does not nurture their quest for transparency, they tend to unconsciously cling to the “majority view” as if their very existence depends on it. The idea of the majority view or the “mainstream”, gives people the sense that they are a part of a group, and at the same time, gives them the illusion of being informed. Their rationale is:If most of the population believes something to be true, then, by “statistical law”, it most likely is true. Those who do not share in the majority opinion are therefore in opposition to statistical law; meaning they are behind the times, social deviants, or just plain crazy…The problem is, history has shown that at pivotal moments in a society the “majority opinion” is usually wrong. Any progress we do enjoy as a species is almost always due to the actions of tireless aware minorities, or even a lone man or woman who saw what the rest of us could not.
Labels:
another magic cure,
bubble,
cognitive,
deconstruction,
failure,
investment,
nice idea,
paradigm,
politics,
real science,
targeted failure
Saturday, January 26, 2013
A. Dugin: Russian dreams
Labels:
cognitive,
deconstruction,
failure,
future foresight,
paradigm,
politics,
quantum,
real science,
semantic,
video
Quote of the day. What is democracy?
которое даёт или пытается дать народу иллюзию того,
что он является господином
© Бенито Муссолини
Labels:
another magic cure,
chaos,
cognitive,
deconstruction,
depression,
failure,
future foresight,
nice idea,
paradigm,
politics,
quantum,
real science,
semantic,
shit of the day
Masterpiece of the day. New reform of aducation in Russia

Labels:
another magic cure,
deconstruction,
future foresight,
masterpiece of the day,
nice idea,
paradigm,
real science,
semantic
Friday, January 25, 2013
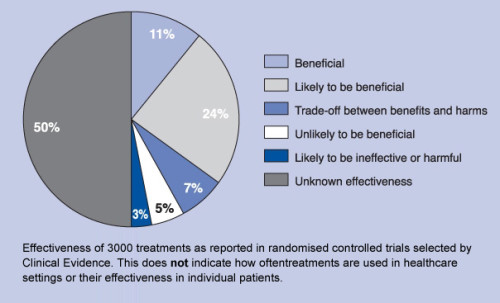
Effectiveness of medical treatments. Surprise?
No, it is
not a surprise – it is natural result of the modern paradigm in health care and
drug industry.
Keep in
mind that the numbers above do not represent how often a given medical
treatment is used. Instead, they represent the number of medical treatments in
each category.
“We want to
identify treatments that work and for which the benefits outweigh the harms,
especially treatments that may be underused,” the authors note. “We also wish
to highlight treatments that do not work or for which harms outweigh benefits.”
Those that
fall into the “unknown effectiveness” category are medical treatments that “for
which there are currently insufficient data or data of inadequate quality.”
When health
policy wonks talk about ending unnecessary care, they usually mean targeting
these types of treatments — the ones where we have no idea whether they’re
making us any healthier. but still increase spending.
Labels:
Big Pharma,
bubble,
chaos,
Death Valley,
deconstruction,
depression,
dirty swan,
evidence based medicine,
failure,
HTS,
numbers,
paradigm,
real science,
shit of the day,
targeted failure
Quote of the day. Francis Bacon

Labels:
another magic cure,
chaos,
cognitive,
deconstruction,
future foresight,
magic,
nice idea,
paradigm,
Partisan of Death Valley,
quantum,
quote of the day,
real science,
semantic
Thursday, January 24, 2013
Capitalism kills innovations. Copy-left. Etc.
Khazin is right!?
Labels:
another magic cure,
bubble,
chaos,
cognitive,
deconstruction,
failure,
future foresight,
macro economy,
nice idea,
numbers,
paradigm,
politics,
quantum,
real science,
shit of the day,
video
Pereslegin. Za-strategija?
Labels:
another magic cure,
chaos,
cognitive,
Death Valley,
deconstruction,
future foresight,
magic,
masterpiece of the day,
nice idea,
paradigm,
Partisan of Death Valley,
politics,
quantum,
real science,
semantic,
video
Quote of the day. Gorky
Labels:
another magic cure,
cognitive,
deconstruction,
failure,
magic,
nice idea,
politics,
real science,
semantic,
shit of the day
Wednesday, January 23, 2013
I like forecasts!

Labels:
chaos,
deconstruction,
depression,
future foresight,
macro economy,
nice idea,
numbers,
paradigm,
politics,
real science
Shit of the day. It's time to say OOOOOOOUT!
Labels:
another magic cure,
chaos,
cognitive,
deconstruction,
depression,
failure,
future foresight,
macro economy,
masterpiece of the day,
nice idea,
politics,
quote of the day,
real science,
shit of the day,
video
Achtung! Achtung! Demura and Khazin
Healthy dose of conspirology
Labels:
chaos,
cognitive,
deconstruction,
depression,
future foresight,
macro economy,
nice idea,
paradigm,
Partisan of Death Valley,
politics,
real science,
shit of the day,
video
Tuesday, January 22, 2013
Another magic cure against everything! Pharma Industry!
Innovation
in the Biopharmaceutical Pipeline: A Multidimensional View. (.pdf is here).
Very interesting reading! The numbers and the scale are amazing!
§ Preclinical research accounted for the
highest number of projects (over 9,000) and potential new medicines (over
6,500). These figures are likely an underestimate, as many preclinical research
activities may not yet have been the subject of news or analyst coverage or may
only be known to the researchers and manufacturers involved, and therefore
would not yet be reflected in the dataset.
§ Over 5,400 new products were in clinical
development (defined in this report as products in Phase I, II, III, or having
been filed with the FDA, or approved by the FDA, but not yet on the market in
the U.S.). Since a single product may be investigated for multiple indications,
and because the data include additional indications for products already
approved and on-market, the number of pipeline projects in clinical development
is larger, or about 8,000.
Consistent with previous studies showing
high attrition rates between Phase II and the much more expensive and lengthy
Phase III clinical trial stage, there were many fewer compounds at each progressive
phase of development. Whereas there were 2,329 molecules recorded in Phase II
clinical trials, there were only 833 products in Phase III trials. A total of
82 products in the dataset had completed Phase III clinical trials and had
either been filed with the FDA or were approved by the FDA, but had not yet
been launched in the U.S.
And the numbers is not the only
thing! There are a lot of innovative products! For sure!
A range of novel scientific
approaches to address various diseases and conditions were being
pursued. Broad classes of
scientific “platforms” readily identifiable in the dataset revealed:
- 245 projects using cell
therapy;
- 127 projects using antisense
RNA interference therapy (an approach that targets RNA, which
carries genetic information that
creates proteins, rather than proteins themselves);
- 102 projects using monoclonal
antibodies joined to cytotoxic agents to target tumor cells
while sparing nearby healthy
cells; and
-
99 projects using gene therapy.
Well, I guess in about 10 years we
will get cured all diseases and people will die absolutely healthy!
Labels:
another magic cure,
Big Pharma,
bubble,
chaos,
cognitive,
deconstruction,
failure,
future foresight,
magic,
nice idea,
numbers,
paradigm,
Partisan of Death Valley,
real science,
shit of the day,
targeted failure
Monday, January 21, 2013
A.I.Fursov. Psycological wars
Labels:
chaos,
cognitive,
deconstruction,
paradigm,
politics,
real science,
semantic,
shit of the day,
video
Definition of the day: Blockbusters
Blockbusters are defined as drugs that generated $1 billion in sales in 2000, which coincides with the generally accepted beginning of the blockbuster era. That amount has been adjusted annually to reflect the CPI inflation, and it stood at $1.34 billion in 2012. (From here)
Labels:
Big Pharma,
deconstruction,
nice idea,
numbers,
paradigm,
real science
Some details of 2012 FDA approvals
Very interesting numbers (from here). It looks like Big Pharma still has a lot of unsolved problems:
Only 13 drugs out of 37 (35%) were licensed to Big Pharma. This is consistent with data from the last 8 years when the share of Big Pharma has hovered between 25% and 40%.
Based on historical data, only about 4 or 5 of these 13 drugs will become blockbusters. The rest will peak on average at about $500 million. This is insufficient to support the sales of the top 13 pharma, which in 2011 where just short of $400 billion. Despite their chief executives’ best intentions, we can expect significant turbulence ahead.
The companies that need new drugs the most did not get them. Since 2005, Abbott, AstraZeneca and Lilly have collectively received 3 NME approvals (excluding imaging agents), a troublesome performance for companies that have historically made major contributions to innovation. By comparison, the top 3 companies, J&J, GSK, and Novartis received 31 approvals during the same period.
The top 13 pharma spend about $72 billion a year on R&D. This is too much for too little output. It lends credence to the CEOs who have argued that the return on pharma R&D is now globally negative, and it is a problem that must be fixed.
However, there is little consensus on how to fix it. Some chief executives have argued for more IP, higher prices, and friendly policies, in other words more of the same, while others have set out to dismantle the regimented R&D culture that forced their creative scientists to do uncreative things. Perhaps the differences between these approaches have started to sort the winners from the losers.
The other question is that new drugs, even those that target new mechanisms, do not necessarily equate with superior outcomes. Some patients (and payers) have been grumbling about treatments that deliver “six months of misery” made more miserable by the price tags they carry. Innovation often gets better when the bar is higher. Perhaps the industry should pay more heed to Jim Watson’s admonitions: “our focus on mediocre drugs keeps us from developing good ones”…”FDA should not approve cancer drugs that provide less than a year of survival benefit”.
Yet, there is much to cheer about the class of 2012. It shows that innovation is resurging across the industry. The capabilities are there, and some leaders are figuring out ways to harness them. This suggests that perhaps there is no innovation crisis at all, only a leadership crisis. Innovation thrives where it is supported by enlightened leadership and shrivels otherwise. Let us hope that there won’t be shriveling in 2013.

Labels:
another magic cure,
bubble,
cognitive,
Death Valley,
deconstruction,
evidence based medicine,
nice idea,
numbers,
paradigm,
Partisan of Death Valley,
real science,
targeted failure
Masterpiece of the day. Not politically correct again

Labels:
another magic cure,
chaos,
cognitive,
deconstruction,
failure,
future foresight,
magic,
masterpiece of the day,
nice idea,
politics,
real science,
semantic,
shit of the day
Sunday, January 20, 2013
The old man.
Zbyszek is too old, does not understand the world... He has no clue about the trends...
Labels:
chaos,
deconstruction,
depression,
failure,
future foresight,
paradigm,
politics,
real science,
shit of the day,
video
Quote of the day. D. Rockfeller
Labels:
chaos,
cognitive,
deconstruction,
depression,
future foresight,
macro economy,
paradigm,
politics,
quote of the day,
real science,
semantic,
shit of the day
Saturday, January 19, 2013
Informational war: made in Poland
It is so funny that I think it has to be fake!




Labels:
chaos,
cognitive,
deconstruction,
depression,
failure,
masterpiece of the day,
politics,
real science,
shit of the day
Quotes of the day. Demura about central bank
Labels:
bubble,
chaos,
cognitive,
deconstruction,
depression,
failure,
future foresight,
paradigm,
politics,
quote of the day,
real science,
shit of the day,
video
A.I.Fursov. Asian revolt
Labels:
chaos,
cognitive,
deconstruction,
future foresight,
nice idea,
paradigm,
politics,
quantum,
real science,
video
Friday, January 18, 2013
ABC of Drug Industry
Labels:
another magic cure,
Big Pharma,
bubble,
chaos,
cognitive,
deconstruction,
depression,
evidence based medicine,
failure,
future foresight,
magic,
nice idea,
paradigm,
real science,
shit of the day,
video
Music of the week. Aleksandryna
...Takoju mnie zdalasia u siamnaccac god maih!..
... Ciabie z nijakaj kvietkaj nie mog ja paraunac...
... Ciabie z nijakaj kvietkaj nie mog ja paraunac...
Labels:
another magic cure,
cognitive,
deconstruction,
magic,
masterpiece of the day,
music of the week,
semantic,
video
Another magic cure against cancer. Post 37-38. Afatinib and Erlotinib
If you still believe in personal medicine (which I do not recommend to do) - this information is right for you! From here.
 Boehringer was
granted priority review for its epidermal growth factor receptor
(EGFR) inhibitor afatinib in NSCLC patients with locally advanced or
metastatic disease who test positive for the EGFR mutation.
Boehringer was
granted priority review for its epidermal growth factor receptor
(EGFR) inhibitor afatinib in NSCLC patients with locally advanced or
metastatic disease who test positive for the EGFR mutation.
Between 10 and 25
per cent of Caucasian NSCLC patients are EGFR-positive, with the
proportion rising to 30-40 per cent in some Asian populations.
Meanwhile, Roche
and Astellas have been given a fast-track review for their EGFR
inhibitor Tarceva (erlotinib) in first-line NSCLC, an indication for
which it was approved in Europe in 2011. Once again approval is being
sought to use the drug in patents with locally advanced or metastatic
NSCLC who are EGFR-positive.
Well, what about
the rest (majority) of the NSCLC which is not EGFR-positive???
Labels:
Big Pharma,
bubble,
chaos,
deconstruction,
future foresight,
nice idea,
numbers,
paradigm,
Partisan of Death Valley,
real science,
targeted failure
Cancer: positive news!
From here.
As of 2009, the overall death rate for
cancer in the United States had declined 20 percent from its peak in
1991, translating to the avoidance of approximately 1.2 million
deaths from cancer, 152,900 of these in 2009 alone.
The authors say as encouraging as those
drops are, further progress can be accelerated by applying existing
cancer control knowledge across all segments of the population, with
an emphasis on those groups in the lowest socioeconomic bracket and
other underserved populations.
According to the study, a total of
1,660,290 new cancer cases and 580,350 cancer deaths are projected to
occur in the United States in 2013. Among men, cancers of the
prostate, lung and bronchus, and colorectum will account for half of
all newly diagnosed cancers; prostate cancer alone will account for
28% (238,590) of incident cases in men. Among women, the three most
commonly diagnosed types of cancer in 2013 will be breast, lung and
bronchus, and colorectum, accounting for about half of all cases.
Breast cancer alone is expected to account for 29% (232,340) of all
new cancer cases among women.
While incidence rates are declining for
most cancer sites, they are increasing among both men and women for
melanoma of the skin and cancers of the liver, thyroid, and pancreas.
Overall cancer incidence rates decreased slightly in males (by 0.6%
per year) and were stable in females in the most recent five year
period for which there is data (2005-2009).
Cancers of the lung and bronchus,
prostate, and colorectum in men and cancers of the lung and bronchus,
breast, and colorectum in women continue to be the most common causes
of cancer death. These four cancers account for almost half of the
total cancer deaths among men and women. In 2013, lung cancer is
expected to account for 26% of all female cancer deaths and 28% of
all male cancer deaths.
Cancer death rates decreased by 1.8%
per year in males and by 1.5% per year in females during the most
recent five years of data (2005-2009). These declines have been
consistent since 2001 and 2002 in men and women, respectively, and
are larger in magnitude than those occurring in the previous decade.
Between 1990/1991 and 2009, cancer death rates decreased by 24% in
men, 16% in women, and 20% overall.
Labels:
another magic cure,
deconstruction,
evidence based medicine,
future foresight,
nice idea,
numbers,
real science
Thursday, January 17, 2013
Fake again?
Labels:
another magic cure,
cognitive,
deconstruction,
magic,
nice idea,
real science,
semantic
Music of the week. Kalina
Magic - for those who understand. Deepest than the ocean, highest than the sky...
Labels:
another magic cure,
cognitive,
deconstruction,
magic,
masterpiece of the day,
music of the week,
real science,
semantic,
video
Wednesday, January 16, 2013
Big Pharma, breast cancer and childish behavior.
From here.
File this under ‘sweep it under the rug.’ It may be human nature to downplay unwanted or negative developments, but it is not considered to be good science. Nonetheless, some investigators have masked disappointing clinical trial results, such as missing primary endpoints and reporting toxicity, with selective and biased reporting for breast cancer treatments, according to a study in the Annals of Oncology.
 Specifically, in one-third of all trials that failed to show a statistically significant benefit for a breast cancer medications being tested, the published studies emphasized less important outcomes in hopes of giving the results a positive spin. However, there was no association between industry or for-profit trial sponsorship and biased reporting of either efficacy or toxicity.
Specifically, in one-third of all trials that failed to show a statistically significant benefit for a breast cancer medications being tested, the published studies emphasized less important outcomes in hopes of giving the results a positive spin. However, there was no association between industry or for-profit trial sponsorship and biased reporting of either efficacy or toxicity.
The researchers examined the results of 164 randomized, controlled Phase III trials of breast cancer treatments that were published between 1995 and 2011. They found 92 that were negative trials in which data did not demonstrate the medication had an effect on the primary endpoint. But in 59 percent of the negative trials, the authors used positive findings from secondary endpoints to cast the treatment in a positive light.
The researchers also found bias in the way adverse effects were discussed. Serious side effects, such as omitting mention in the abstract or conclusion, were found in 67 percent of the publications with both positive and negative findings. Moreover, trials with positive findings were twice as likely to underplay serious adverse effects compared with publications of negative studies.
Labels:
Big Pharma,
chaos,
Death Valley,
deconstruction,
depression,
failure,
paradigm,
Partisan of Death Valley,
real science,
shit of the day
Tuesday, January 15, 2013
The dictator? Do we need more of this type of dictatorship!
And yet another healthy dose of propaganda
Labels:
chaos,
cognitive,
future foresight,
nice idea,
politics,
real science,
video
Berlusconi vs Sarkozy 1:0
Healthy dose of propaganda. I like Berlusconi - a lot of drive for his age!
Labels:
chaos,
cognitive,
deconstruction,
depression,
failure,
macro economy,
paradigm,
politics,
real science,
shit of the day,
video
Targeted failure of the week. Post No 44-45. Farletuzumab and Tredaptive
Eisai
Co. Ltd. (Tokyo:4523; Osaka:4523) said IV farletuzumab plus
carboplatin and taxane chemotherapy missed the primary endpoint of
improving progression-free survival (PFS) vs. placebo plus
carboplatin and taxane chemotherapy in the Phase III FAR 131 trial to
treat platinum-sensitive epithelial ovarian cancer in first relapse.
The double-blind, international trial enrolled 1,100 patients. Eisai,
which said a post hoc exploratory analysis showed a trend toward
improved PFS in undisclosed patient subgroups, said it will determine
a new development strategy for farletuzumab based on discussion with
external experts and health authorities.
With tredaptive the
situation is also very sad:
Less
than a month ago, the company presented data from the HPS2-THRIVE
study of Tredaptive (extended-release niacin/laropiprant) which
enrolled 25,673 patients considered to be at high risk for
cardiovascular events. Data showed that adding the pill to statin
therapy did not significantly further cut the risk of the combination
of coronary deaths, non-fatal heart attacks, strokes or
revascularisations.
There
was also a statistically significant increase in the incidence of
some non-fatal serious side effects in the Tredaptive group, Merck
said at the time. Because of the data, the company has decided not to
seek approval in the USA and is now withdrawing the drug from 40
countries.
The
decision to suspend the medicine came as the European Medicine
Agency’s Pharmacovigilance Risk Assessment Committee also called
for Tredaptive to be withdrawn after a review of the HPS2-THRIVE
data.
Labels:
Big Pharma,
bubble,
deconstruction,
failure,
shit of the day,
targeted failure
Subscribe to:
Posts (Atom)