Thursday, February 27, 2014
Wednesday, February 26, 2014
Targeted failure of the week. Post No 136. Ridaforolimus
 Merck & Co. Inc. (NYSE:MRK) has officially washed its hands of a once-lauded experimental cancer drug it invested in along with Ariad Pharmaceuticals Inc. (NASDAQ:ARIA) Merck informed Ariad last week that it was terminating its license agreement and handing back the drug to Ariad, FierceBiotech reports.
Merck & Co. Inc. (NYSE:MRK) has officially washed its hands of a once-lauded experimental cancer drug it invested in along with Ariad Pharmaceuticals Inc. (NASDAQ:ARIA) Merck informed Ariad last week that it was terminating its license agreement and handing back the drug to Ariad, FierceBiotech reports.At one time, the drug, known as ridaforolimus, was considered a promising new cancer treatment. Merck originally planned to invest up to $700 million in the project, which yielded a phase 3 clinical trial in advanced sarcomas. The drug was then rejected, however, when the Food and Drug Administration claimed that due to the drug’s risk-benefit profile, it would require another trial. Prior to the drug’s rejection by the FDA, Merck had already paid Ariad about $200 million as part of the partnership.
From here:
http://wallstcheatsheet.com/business/stock-news/merck-abandons-once-promising-experimental-cancer-drug-to-ariad.html/
Labels:
Big Pharma,
bubble,
deconstruction,
depression,
evidence based medicine,
failure,
investment,
numbers,
real science,
shit of the day,
targeted failure
Tuesday, February 25, 2014
Masterpiece of creativity

Labels:
chaos,
cognitive,
deconstruction,
masterpiece of the day,
nice idea
Monday, February 24, 2014
Sunday, February 23, 2014
Ukraine has to be free!

Labels:
another magic cure,
cognitive,
deconstruction,
depression,
failure,
future foresight,
masterpiece of the day,
paradigm,
politics,
real science,
targeted failure
Saturday, February 22, 2014
Puzzle your wife!

Labels:
chaos,
cognitive,
deconstruction,
failure,
masterpiece of the day,
real science
Friday, February 21, 2014
Ukraine has to be free!

Labels:
chaos,
cognitive,
deconstruction,
depression,
failure,
future foresight,
politics,
real science,
shit of the day
Wednesday, February 19, 2014
Shit of the day? Not for Finland!

Labels:
cognitive,
deconstruction,
failure,
shit of the day
Nexavar - let's be honest!
Labels:
another magic cure,
Big Pharma,
deconstruction,
failure,
future foresight,
nice idea,
numbers,
paradigm,
politics,
real science
Tuesday, February 18, 2014
Bill Geits advices. The masterpiece of understanding.
Labels:
chaos,
cognitive,
deconstruction,
masterpiece of the day,
nice idea,
paradigm,
real science
Monday, February 17, 2014
Sunday, February 16, 2014
Saturday, February 15, 2014
Friday, February 14, 2014
Thursday, February 13, 2014
Electroceuticals - WTF?
Labels:
another magic cure,
Big Pharma,
cognitive,
deconstruction,
future foresight,
nice idea,
real science
Tuesday, February 11, 2014
Quote of the day. Pereslegin

Считается, что мышление, разум не приемлют насилия. В действительности и то и другое — инструмент насилия: видового, национального, группового, личного. Мы используем свой разум для того, чтобы реализовать свои цели, добиться для себя преимуществ [...] Не нужно различать разум как эволюционный приспособительный механизм, предназначенный, прежде всего, для выигрыша в конкурентной борьбе, и разум как высшую когнитивную способность, обеспечивающую познание, озарение, творчество. Это — одно и то же качество. Просто, когда вы имеете дело с познанием — все равно, в форме творчества или рафинированного научного мышления, — вы сталкиваетесь с самым сильным противником и играете на предельную ставку.
Monday, February 10, 2014
Fake?

Labels:
chaos,
cognitive,
deconstruction,
failure,
masterpiece of the day,
politics,
real science,
shit of the day,
targeted failure
Sunday, February 9, 2014
Saturday, February 8, 2014
Masterpiece of ...

Labels:
chaos,
deconstruction,
failure,
real science,
shit of the day,
targeted failure
Friday, February 7, 2014
AZ: Targeted failure of the industry!
As AstraZeneca was trumpeting the expansion of its late-stage pipeline on Thursday, the pharma giant ($AZN) also unceremoniously swept 15 therapeutic programs out of its pipeline--including a notable mid-stage therapy for depression along with a separate program for pain--leaving behind a shrinking group of neuroscience projects and little by way of explanation on the significance of the clinical executions.
 In a section of discontinued projects for the year, AstraZeneca included AZD6765, one of the "Special K" experimental projects which had been mounted to see if a therapy could be devised that would provide the antidepressive benefit of the party drug without the party. The ketamine-like drug had been highlighted for its ability to trigger a rapid response among patients suffering from depression. But the effect could be ephemeral.
In a section of discontinued projects for the year, AstraZeneca included AZD6765, one of the "Special K" experimental projects which had been mounted to see if a therapy could be devised that would provide the antidepressive benefit of the party drug without the party. The ketamine-like drug had been highlighted for its ability to trigger a rapid response among patients suffering from depression. But the effect could be ephemeral.
A couple of months ago FierceBiotech was tipped off that the drug had failed the second of two Phase II studies and was being dumped--though a spokesperson for the company declined to confirm or deny the report, noting that the update today would include the latest list of Phase I/Phase II fatalities.
In today's list of discontinued projects, AstraZeneca notes only that the depression drug failed for both safety and efficacy. "The results from the Phase IIb PURSUIT study for AZD6765, an adjunctive treatment of major depressive disorder in treatment-refractory patients, did not meet the primary endpoint," says a company spokesperson in an email. "A complete analysis and understanding of PURSUIT results together with previous clinical data will inform future development decisions."
The discards also include MEDI-5117 for pain. About a year ago, MedImmune chief Bahija Jallal had highlighted a deal with WuXi PharmaTech to develop 5117 for rheumatoid arthritis in China as an important initiative. Heralded back in the fall of 2012, AstraZeneca retained half the rights to 5117 and had the option to buy back all the rights in the deal, which was intended to carve a shortcut to the Asian market.
"We are still very much pursuing the development of MEDI-5117 for rheumatoid arthritis through our joint venture with WuXi AppTec," says a spokesperson.
AstraZeneca's reason for discontinuing the pain program: "Safety/efficacy."
The dud list includes AZD8330 (Array's ARRY-424704) an MEK 1/2 inhibitor that was in a Phase I study back in 2009; MEDI-557 for RSV; MEDI-575, which had been in a Phase II for glioblastoma multiforme and evidently non-small cell lung cancer.
Fostamatinib for rheumatoid arthritis was publicly dismissed by AstraZeneca last year, but several other lesser known drugs were written off in the respiratory field: AZD5423, AZD7594 and MED-7814 for COPD, as well as MEDI-4212 for asthma. MEDI-7814 didn't make it for "economic" reasons.
There was also a set of three Alzheimer's therapies, AZD1446, AZD3480, AZD5213, which AstraZeneca had publicly handed back to Targacept ($TRGT) last spring. AZD1446 targets the alpha4beta2 neuronal nicotinic receptor and came out of the research collaboration between Targacept and AstraZeneca. That program was advanced after a major late-stage program for a depression drug was scuttled by bad data, raising some serious questions about Targacept's scientific approach and AstraZeneca's knack for picking losers.
AstraZeneca says it's adopting a new approach to burying the dead. It will do it quarterly now rather than every 6 months.
Labels:
Big Pharma,
bubble,
chaos,
cognitive,
deconstruction,
evidence based medicine,
failure,
HTS,
nice idea,
numbers,
paradigm,
real science,
targeted failure
Thursday, February 6, 2014
Prognosis
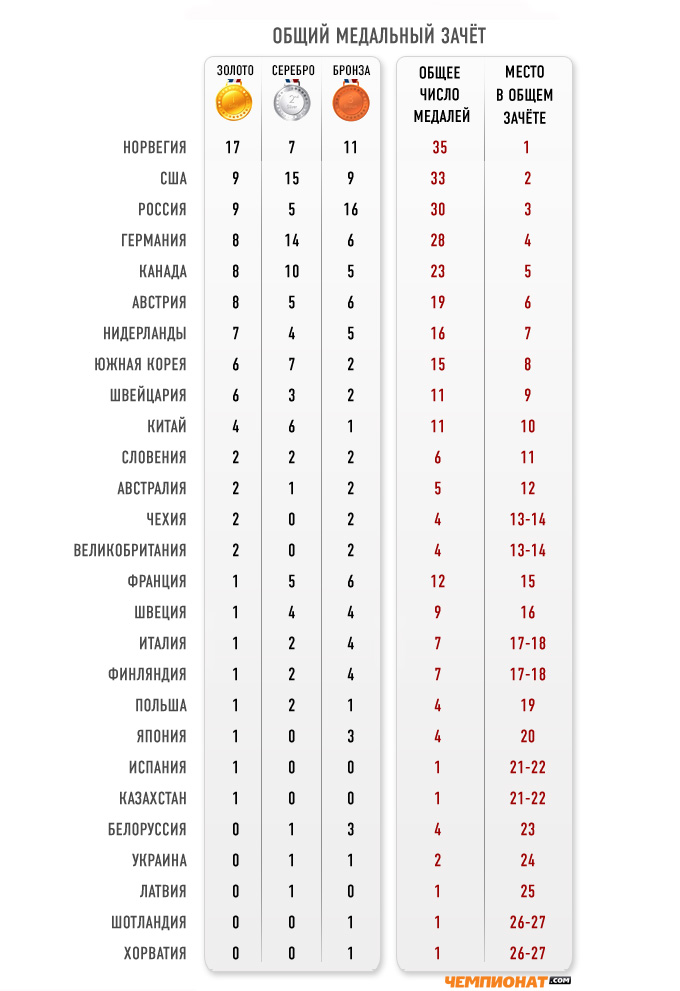
Labels:
chaos,
cognitive,
deconstruction,
failure,
future foresight,
nice idea,
real science,
shit of the day,
targeted failure
Wednesday, February 5, 2014
Tuesday, February 4, 2014
Ukraine - tse Europa. This country must be free!

Labels:
another magic cure,
chaos,
cognitive,
Death Valley,
deconstruction,
failure,
future foresight,
masterpiece of the day,
nice idea,
paradigm,
Partisan of Death Valley,
politics,
real science,
targeted failure
Monday, February 3, 2014
Sunday, February 2, 2014
Saturday, February 1, 2014
Subscribe to:
Posts (Atom)















