Saturday, June 29, 2013
Targeted failure of the week. Post No 88. RVX-208
From here.
Resverlogix Corp. (RVX) sank the most on record after saying its drug to fight thickening of the arteries failed to meet its primary target in a phase 2 clinical trial.
Resverlogix, based in Calgary, plunged 93 percent to 23 cents at 4 p.m. in Toronto, the most since its initial public offering in 2001. The stock has now fallen 86 percent this year, giving it a market value of C$17.2 million ($16.4 million).
Test results from a 26-week Phase 2b clinical trial showed patients treated with the RVX-208 drug had a 0.4 percent reduction in plaque levels, short of the 0.6 percent target drop.
“We remain focused on analyzing the full data set over the coming weeks and months to determine whether continued development of RVX-208 in cardiovascular disease is warranted,” Donald McCaffrey, Resverlogix’s chief executive officer, said in a statement.
The drug is intended to work by boosting production of a particle required in a process called reverse cholesterol transport, in which plaque in the arteries is removed from the body using the liver.
Well, the drug has a very sexy story: “RVX 208 represents a potentially novel class of medication that can drive the formation of nascent HDL particles in humans, increase HDL particle numbers … and promote increased cellular cholesterol efflux.” What is wrong with the drug??? Or probably the story is not correct???
Resverlogix Corp. (RVX) sank the most on record after saying its drug to fight thickening of the arteries failed to meet its primary target in a phase 2 clinical trial.
Resverlogix, based in Calgary, plunged 93 percent to 23 cents at 4 p.m. in Toronto, the most since its initial public offering in 2001. The stock has now fallen 86 percent this year, giving it a market value of C$17.2 million ($16.4 million).
Test results from a 26-week Phase 2b clinical trial showed patients treated with the RVX-208 drug had a 0.4 percent reduction in plaque levels, short of the 0.6 percent target drop.
“We remain focused on analyzing the full data set over the coming weeks and months to determine whether continued development of RVX-208 in cardiovascular disease is warranted,” Donald McCaffrey, Resverlogix’s chief executive officer, said in a statement.
The drug is intended to work by boosting production of a particle required in a process called reverse cholesterol transport, in which plaque in the arteries is removed from the body using the liver.
Well, the drug has a very sexy story: “RVX 208 represents a potentially novel class of medication that can drive the formation of nascent HDL particles in humans, increase HDL particle numbers … and promote increased cellular cholesterol efflux.” What is wrong with the drug??? Or probably the story is not correct???
Labels:
Big Pharma,
bubble,
Death Valley,
deconstruction,
depression,
evidence based medicine,
failure,
real science,
shit of the day,
targeted failure
Subcutaneous trastuzumab?
I would propose to try inhalation drug delivery. Or rectal drug administration for trastuzumab - which for sure would be even less invasive.

Friday, June 28, 2013
Targeted failure of the week. Post No 87. Bavituximab
This time is just another mAb:
Shares of Peregrine Pharmaceuticals Inc. sank Thursday after the drug developer said it will stop studying a treatment combination involving its potential lung cancer drug bavituximab.
THE SPARK: The Tustin, Calif., company said a combination of bavituximab, carboplatin and a chemotherapy drug labeled paclitaxel did not produce a meaningful enough difference in overall survival in patients compared with carboplatin and paclitaxel alone. The company had just completed an analysis of data from a mid-stage study of the drug.
Shares of Peregrine Pharmaceuticals Inc. sank Thursday after the drug developer said it will stop studying a treatment combination involving its potential lung cancer drug bavituximab.
THE SPARK: The Tustin, Calif., company said a combination of bavituximab, carboplatin and a chemotherapy drug labeled paclitaxel did not produce a meaningful enough difference in overall survival in patients compared with carboplatin and paclitaxel alone. The company had just completed an analysis of data from a mid-stage study of the drug.
Labels:
Big Pharma,
chaos,
Death Valley,
depression,
evidence based medicine,
failure,
paradigm,
shit of the day,
targeted failure
Thursday, June 27, 2013
Spendings on pharmaceuticals. Numbers...
Labels:
Big Pharma,
deconstruction,
depression,
numbers,
real science,
shit of the day
Wednesday, June 26, 2013
Targeted failure of the week. Post No 86.Brentuximab vedotin
A small clinical study treating elderly Hodgkin lymphoma patients with a combination of Seattle Genetics' (SGEN_) Adcetris and chemotherapy has been stopped temporarily due to reports of pancreatitis, a dangerous swelling of the pancreas, the company confirmed Tuesday.
The decision to suspend patient enrollment in the Adcetris study due to concerns about pancreatitis was made by researchers at Chicago's Northwestern University, which designed and is conducting the study. Seattle Genetics says none of its Adcetris studies have been halted for any toxicity reasons.
The decision to suspend patient enrollment in the Adcetris study due to concerns about pancreatitis was made by researchers at Chicago's Northwestern University, which designed and is conducting the study. Seattle Genetics says none of its Adcetris studies have been halted for any toxicity reasons.
Yes, antibody-drug conjugate (ADC) being extremely "sexy" "magic bullet" fails... Was it expected for those who understand all this targeted bogus??? For sure! More failures to come!
Labels:
bubble,
chaos,
deconstruction,
depression,
failure,
future foresight,
real science,
shit of the day,
targeted failure
10 top drug launch disasters
10 top drug launch disasters - interesting reading regarding:
1
K-V Pharmaceuticals - Makena
2
Dendreon - Provenge
3
Sanofi - Zaltrap
4
Human Genome Sciences - Benlysta
5
Xenoport - Horizant
6
Savient - Krystexxa
7
Sanofi - Multaq
8
Somaxon - Silenor
9
AstraZeneca - Brilinta
10
Rare Disease Therapeutics - Anascorp
1
K-V Pharmaceuticals - Makena
2
Dendreon - Provenge
3
Sanofi - Zaltrap
4
Human Genome Sciences - Benlysta
5
Xenoport - Horizant
6
Savient - Krystexxa
7
Sanofi - Multaq
8
Somaxon - Silenor
9
AstraZeneca - Brilinta
10
Rare Disease Therapeutics - Anascorp
Labels:
Big Pharma,
chaos,
Death Valley,
deconstruction,
depression,
evidence based medicine,
failure,
paradigm,
real science,
shit of the day
Tuesday, June 25, 2013
Sunday, June 23, 2013
Friday, June 21, 2013
Thursday, June 20, 2013
Targeted failure of the week. Post No 85.Saxagliptin
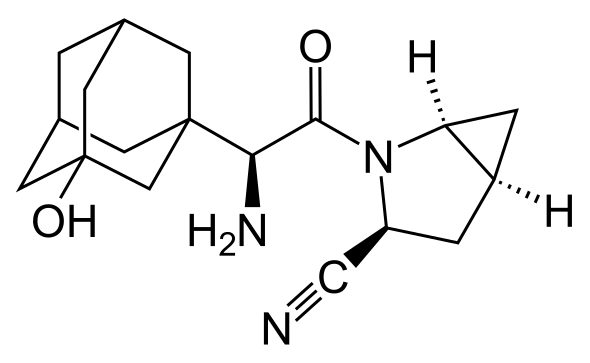 The companies, which jointly sell several diabetes drugs, said on Wednesday the SAVOR trial showed that patients on Onglyza had no fewer adverse cardiovascular events, such as heart attacks and strokes, than those on placebo.
The companies, which jointly sell several diabetes drugs, said on Wednesday the SAVOR trial showed that patients on Onglyza had no fewer adverse cardiovascular events, such as heart attacks and strokes, than those on placebo.
Doctors and investors had been awaiting results of the SAVOR trial with keen interest, since a positive result could have encouraged increased use of the drug and others from its class, which also includes Merck & Co's Januvia, the market leader.
Labels:
Big Pharma,
bubble,
deconstruction,
depression,
evidence based medicine,
failure,
real science,
targeted failure
Tuesday, June 18, 2013
Targeted failure of the week.Post No 84.LY2886721
The latest drug to bite the dust is LY2886721, a beta secretase, or BACE, inhibitor. Eli Lilly stopped a phase 2 trial after some patients had abnormal liver biochemical tests. Companies routinely run liver tests on clinical trial patients because it's one of the most common safety issues that affect drugs. The liver's job, after all, is to filter toxins out of the blood stream.
Labels:
Big Pharma,
deconstruction,
depression,
failure,
HTS,
paradigm,
shit of the day,
targeted failure
Monday, June 17, 2013
Sunday, June 16, 2013
Saturday, June 15, 2013
Friday, June 14, 2013
Masterpiece of the day.

Labels:
cognitive,
masterpiece of the day,
nice idea,
real science
Thursday, June 13, 2013
Wednesday, June 12, 2013
Can be discussed

Labels:
cognitive,
deconstruction,
failure,
masterpiece of the day,
real science
Tuesday, June 11, 2013
Targeted failures of the week. Post No 82 and 83. Tykerb and Tivozanib

The randomized, multi-center, double-blinded, phase III study compared the efficacy and tolerability of Tykerb as an adjunct to Sanofi’s Eloxatin (oxaliplatin) and Roche Holding’s Xeloda (capecitabine) versus Eloxatin, Xeloda and placebo in treatment naïve HER2-positive advanced gastric cancer patients.
Results from the study revealed that the treatment with Tykerb did not cause significant improvement in overall survival (:OS), thus failing to meet the primary endpoint of the study. The median OS was 12.2 months in the Tykerb plus Eloxatin and Xeloda arm compared with 10.5 months for the Eloxatin, Xeloda and placebo arm.
In a complete response letter to the company, the health regulator said inconsistent survival data from the drug tivozanib's trials made the results "uninterpretable and inconclusive."
What's wrong with the World? Why Big Pharma produces targeted drugs which are wrong-targeted?
Monday, June 10, 2013
WTF of the day: Big Pharma. The conspirology
Labels:
another magic cure,
Big Pharma,
deconstruction,
depression,
failure,
macro economy,
nice idea,
numbers,
paradigm,
real science,
shit of the day,
video
Conspirology is BS!

Labels:
another magic cure,
cognitive,
deconstruction,
future foresight,
nice idea,
paradigm,
politics,
real science
Sunday, June 9, 2013
DM... Again???
Labels:
another magic cure,
cognitive,
deconstruction,
evidence based medicine,
music of the week,
nice idea,
real science,
semantic,
video
Saturday, June 8, 2013
Liberal pornography in two parts. Atlas Shrugged. For 18+
Labels:
another magic cure,
chaos,
deconstruction,
depression,
future foresight,
macro economy,
shit of the day,
targeted failure,
video
Go long or short? Place your bet!
Labels:
Big Pharma,
deconstruction,
evidence based medicine,
future foresight,
investment,
nice idea,
numbers,
real science
Sochi 2014
.jpg)
Labels:
cognitive,
deconstruction,
future foresight,
masterpiece of the day,
nice idea,
paradigm,
politics,
real science,
shit of the day
Friday, June 7, 2013
News of the day!

Labels:
deconstruction,
future foresight,
nice idea,
politics,
real science
Greed kills
From here.
No doubt about it--cancer drugs are hot. With new targeted therapies coming online--and immunotherapies the talk of this year's ASCO meeting--oncologists are getting new treatments for their tool chests, and drugmakers are adding lucrative products to their cash boxes.
But there's a hitch: cost. These new cancer therapies are potentially lucrative because they're pricey. Some recent rollouts top $100,000 per year, with upper-6-figure prices for the rest. The highly anticipated immunotherapies are expected to run around $110,000 in the U.S., analysts tell Reuters, with $80,000 price tags common in the rest of the world.
So, experts say, cancer care will have to change. In fact, it already has. One recently launched cancer drug, Sanofi's ($SNY) Zaltrap, was immediately discounted after Memorial Sloan-Kettering doctors decided its benefits weren't worth the price of about $10,000 per month. The top cancer hospital may do the same for other pricey drugs, doctors said. Meanwhile, insurers are prereviewing drug therapies more often, and they're less inclined to approve off-label use. And as Reuters notes, some 80% of U.S. insurers told PricewaterhouseCoopers they won't add new therapies to their formularies without evidence of cost savings and clinical benefits.
Labels:
Big Pharma,
bubble,
deconstruction,
failure,
future foresight,
numbers,
paradigm,
real science,
shit of the day
Despite Spending $50 Billion Per Year In R&D, Pharma's New Drugs Less Effective Than Drugs Developed 40 Years Ago
From here
 From 2000-2007, 667 new drugs were approved by the FDA. Of those, only 75 (11%) were new molecules that were much better than what we already had. In fact, over 80% of all drugs approved were no better than what we already had. Those are "me-too" drugs. Why do the pharmaceutical companies spend so much on marketing? Because you have to really promote drugs that really have no benefit over others that already exist. You have to convince people to buy those.
From 2000-2007, 667 new drugs were approved by the FDA. Of those, only 75 (11%) were new molecules that were much better than what we already had. In fact, over 80% of all drugs approved were no better than what we already had. Those are "me-too" drugs. Why do the pharmaceutical companies spend so much on marketing? Because you have to really promote drugs that really have no benefit over others that already exist. You have to convince people to buy those.
You know what needs no promotion? Awesome new drugs that save lives. When was the last time you saw a commercial for chemotherapy? For epinephrine? For steroids? Those drugs need no promotion - doctors just know to use them. But I bet all of you know about Nexium. Or Cialis.
 From 2000-2007, 667 new drugs were approved by the FDA. Of those, only 75 (11%) were new molecules that were much better than what we already had. In fact, over 80% of all drugs approved were no better than what we already had. Those are "me-too" drugs. Why do the pharmaceutical companies spend so much on marketing? Because you have to really promote drugs that really have no benefit over others that already exist. You have to convince people to buy those.
From 2000-2007, 667 new drugs were approved by the FDA. Of those, only 75 (11%) were new molecules that were much better than what we already had. In fact, over 80% of all drugs approved were no better than what we already had. Those are "me-too" drugs. Why do the pharmaceutical companies spend so much on marketing? Because you have to really promote drugs that really have no benefit over others that already exist. You have to convince people to buy those. You know what needs no promotion? Awesome new drugs that save lives. When was the last time you saw a commercial for chemotherapy? For epinephrine? For steroids? Those drugs need no promotion - doctors just know to use them. But I bet all of you know about Nexium. Or Cialis.
Thursday, June 6, 2013
Propaganda of the day. Just more music!

Labels:
cognitive,
deconstruction,
depression,
future foresight,
masterpiece of the day,
nice idea,
politics,
real science,
semantic,
shit of the day
Wednesday, June 5, 2013
Nano Shit of the Day. Nano-BS
Labels:
deconstruction,
depression,
evidence based medicine,
nanotechnology,
real science,
shit of the day
Tuesday, June 4, 2013
Chinese quote of the day
Labels:
cognitive,
deconstruction,
masterpiece of the day,
nice idea,
paradigm,
politics,
quote of the day,
real science
Targeted failures of the week. Post No 75 - 81. Iniparib, Otamixaban, Alimta, Cetuximab, GemVax pancreatic cancer vaccine and Ganetespib
This week is a something regarding the amount of failed drug candidate. And almost all failures are "targeted" medicines:
 In the Iniparib study, newly diagnosed cell lung patients were treated with the drug in conjunction with chemotherapy. According to Sanofi, "There were no clinically meaningful differences in the main safety parameters," compared to patients who received chemotherapy alone. Ending development of Iniparib will result in a $235 million non-cash charge recognized on Sanofi's June 30, 2013 consolidated balance sheet.
In the Iniparib study, newly diagnosed cell lung patients were treated with the drug in conjunction with chemotherapy. According to Sanofi, "There were no clinically meaningful differences in the main safety parameters," compared to patients who received chemotherapy alone. Ending development of Iniparib will result in a $235 million non-cash charge recognized on Sanofi's June 30, 2013 consolidated balance sheet. The results for the anticoagulant Otamixaban were also unsuccessful and the drug did not "meet its primary endpoint of superiority over current therapy," according to Sanofi. Following the study, it was determined that the investigational program for Otamixaban will also be discontinued.
The results for the anticoagulant Otamixaban were also unsuccessful and the drug did not "meet its primary endpoint of superiority over current therapy," according to Sanofi. Following the study, it was determined that the investigational program for Otamixaban will also be discontinued. According to the company, the Pronounce study did not achieve its primary superiority endpoint of improved progression-free survival without grade four adverse events or G4PFS. The safety and efficacy profile for Alimta has been the subject of Lilly-sponsored studies involving an estimated 32,500 patients over the span of about 20 years.
According to the company, the Pronounce study did not achieve its primary superiority endpoint of improved progression-free survival without grade four adverse events or G4PFS. The safety and efficacy profile for Alimta has been the subject of Lilly-sponsored studies involving an estimated 32,500 patients over the span of about 20 years.
The study revealed no significant difference between the treatment arms for secondary endpoints of progression-free survival or PFS overall survival, overall response rate and disease control rate. Toxicity profiles observed were consistent with the known safety profiles of each therapy.
The Pronounce trial compared Alimta, carboplatin doublet regimen to a paclitaxel, carboplatin and bevacizumab triplet regimen.
Pronounce is a randomized, open-label Phase III superiority study of first-line chemotherapy pemetrexed plus carboplatin, followed by maintenance pemetrexed, compared to paclitaxel plus carboplatin plus bevacizumab, followed by maintenance bevacizumab in patients with advanced non-squamous NSCLC conducted in the U.S.
Merck KGaA said first-line treatment with Erbitux cetuximab plus FOLFIRI chemotherapy missed the primary endpoint of significantly improving objective response rate (ORR) ORR vs. Avastin bevacizumab plus FOLFIRI chemotherapy (62% vs. 58%, p=0.183) in the Phase III FIRE-3 trial to treat colorectal cancer. On secondary endpoints, median progression-free survival (PFS) was similar between the Erbitux and Avastin arms (10 vs. 10.3 months), but median overall survival (OS) was significantly improved in the Erbitux vs. Avastin arm (28.7 vs. 25 months, p=0.017). The University of Munich sponsored the open-label trial, which enrolled 592 patients with wild-type K-Ras colorectal cancer. Data were presented at the American Society of Clinical Oncology meeting in Chicago.
The efficacy of Synta Pharmaceuticals' lung cancer drug ganetespib is weakening over time, which should raise even more concerns about the ongoing phase III clinical trial.
Labels:
deconstruction,
depression,
failure,
paradigm,
Partisan of Death Valley,
real science,
shit of the day,
targeted failure
Monday, June 3, 2013
War according Dugin
Labels:
cognitive,
deconstruction,
nice idea,
politics,
real science,
video
Targeted failure of the week. Post No 74. Avastin again!
Yes, Avastin failed again. This time for brain tumor:


The widely used Roche Holding AG cancer drug Avastin failed to prolong survival when added to chemo-radiation therapy for glioblastoma - a fast-growing type of brain tumor - according to data presented on Sunday.
Those who received Avastin in the late-stage study of 637 previously untreated patients also experienced more side effects, such as low platelet counts, blood clots and elevated blood pressure. Researchers said the toxicity was not severe enough to preclude use of Avastin, or bevacizumab, had it helped patients live longer.
Labels:
deconstruction,
failure,
real science,
shit of the day,
targeted failure
Pryhazosc
Labels:
another magic cure,
cognitive,
nice idea,
politics,
real science,
video
Sunday, June 2, 2013
Saturday, June 1, 2013
Masterpiece of tax optimization
Labels:
another magic cure,
cognitive,
Death Valley,
deconstruction,
future foresight,
investment,
macro economy,
magic,
masterpiece of the day,
nice idea,
numbers,
paradigm,
politics,
real science
More rocket science on liver: Liver Malignancy Cryosurgery
The Music should be changed to "Let it be" (Beatles)
Labels:
another magic cure,
cognitive,
deconstruction,
depression,
paradigm,
real science,
video
Subscribe to:
Posts (Atom)