Thursday, February 28, 2013
Development Success Rates. NME. Fresh numbers
Labels:
another magic cure,
deconstruction,
depression,
evidence based medicine,
failure,
HTS,
nice idea,
numbers,
paradigm,
real science,
shit of the day
Shit of the day. Painful sex in USA
Are you ready for news regarding new drug? Approved by FDA!? Here it is: Osphena! No jokes - the drug is approved to treat women experiencing pain during sexual intercourse.
The approval is based on three studies of 1,889 postmenopausal women who were
randomly assigned to receive Osphena or placebo. After 12 weeks of treatment,
results from the first two trials showed a statistically significant improvement
of dyspareunia in Osphena-treated women and data from the third 52-week study
support its long-term safety.
Osphena's green light comes with a boxed warning which notes that the drug
can stimulate the lining of the uterus and cause it to thicken, which is not
normal in ostmenopausal women. Patients should see their doctor if they
experience any unusual bleeding as it may be a sign of endometrial cancer or a
condition that can lead to it. The warning also states the risk of strokes and
deep vein thrombosis.
Shionogi quoted David Portman, director of the Columbus Center for Women's
Health Research in Ohio, as saying that while more than half of all women in the
USA will experience symptoms of VVA at some time in their postmenopausal life,
"the vast majority…are not being treated with a prescription medication because
women and their healthcare professionals are not proactively discussing the
condition, and its associated symptoms". He added that as an oral medication
taken once-daily, "Osphena is a convenient way for postmenopausal women to help
treat dyspareunia".
VVA is currently treated with vaginally inserted tablets, creams or rings,
which Shionogi has noted can be inconvenient or messy. The Japan-headquartered
drugmaker obtained the rights to Osphena from the USA's QuatRx Pharmaceuticals
in 2010.
Well, everything looks rational. I think that the US market is right one for this drug!
Labels:
another magic cure,
Big Pharma,
bubble,
chaos,
deconstruction,
future foresight,
masterpiece of the day,
nice idea,
paradigm,
real science,
shit of the day
Wednesday, February 27, 2013
Information aggression. ABC.
Labels:
another magic cure,
deconstruction,
depression,
nice idea,
paradigm,
politics,
real science,
semantic,
shit of the day,
video
Masterpiece of the day. The real liberal economist!
Labels:
another magic cure,
cognitive,
deconstruction,
failure,
masterpiece of the day,
nice idea,
politics,
real science,
semantic,
shit of the day
Tuesday, February 26, 2013
Another targeted failure of the week. Post No 53. Cilengitide
 Merck KGaA's Cilengitide, an experimental drug to treat an aggressive type of brain tumor, has failed a large-scale clinical trial, dealing a blow to the German drugmaker's efforts to replenish its pipeline of medicines.
Merck KGaA's Cilengitide, an experimental drug to treat an aggressive type of brain tumor, has failed a large-scale clinical trial, dealing a blow to the German drugmaker's efforts to replenish its pipeline of medicines.
Merck said on Monday patients in a Phase III trial did not live significantly longer when treated with Cilengitide plus chemoradiotherapy.
Dr. Annalisa Jenkins, Head of Global Drug Development and Medical for the Merck Serono division, said the trial results were "disappointing, especially for people who are fighting this devastating and difficult to treat cancer".
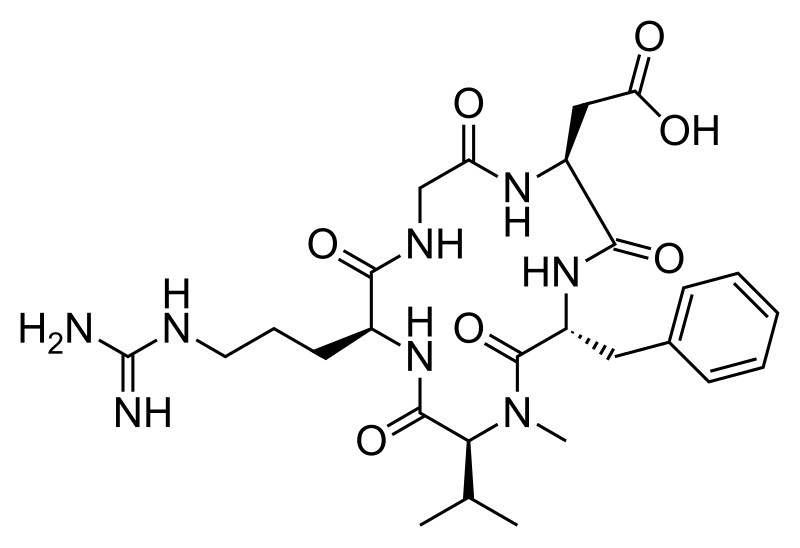
And Cilengitide is a targeted drug. It is based on the cyclic peptide cyclo(-RGDfV-), which is selective for αv integrins, which are important in angiogenesis (forming new blood vessels).
New week and a new failure of targeted drugs...
Labels:
chaos,
depression,
failure,
real science,
shit of the day,
targeted failure
Shit of the day. Ateism
Labels:
chaos,
cognitive,
deconstruction,
failure,
nice idea,
paradigm,
real science,
shit of the day
Monday, February 25, 2013
Sunday, February 24, 2013
A.I.Fursov. Criminalization of elite. (Shit of the day)
Labels:
chaos,
cognitive,
deconstruction,
depression,
failure,
future foresight,
macro economy,
nice idea,
paradigm,
politics,
quantum,
shit of the day,
video
Masterpiece of the day. How to change russian government
Labels:
deconstruction,
failure,
future foresight,
masterpiece of the day,
nice idea,
paradigm,
politics,
real science,
shit of the day
Shit of the day. Fake?
Labels:
chaos,
cognitive,
depression,
future foresight,
nice idea,
paradigm,
politics,
real science,
shit of the day
Saturday, February 23, 2013
Shit of the propaganda. Condoms do not protect you.
Labels:
Big Pharma,
Death Valley,
deconstruction,
depression,
evidence based medicine,
failure,
nice idea,
politics,
real science,
shit of the day,
targeted failure,
video
Friday, February 22, 2013
Epigenetic Therapies and Technologies: WTF???
Well, I do not know how to present the information regarding epigenetic therapies and technologies that I have obtained as an advertisement in my e-mail address... But several quotes to start:
Research and development - see trends and possibilities
What's happening in epigenetic R&D? You see trends and outlooks there:
• HDAC inhibitors
• DNMT inhibitors
• Treatments targeting other enzymes
• MiRNA therapy
• Diagnostics pipeline.
Our study also discusses these technologies, among others:
• New drug targets
• Biomarkers
• Bioinformatics
• Next generation sequencing.
Discover progress. You assess innovations affecting the industry's future - hear about developments and find their significance. Our work explains, exploring many issues.
What affects the application of epigenetics?
 Our report discusses issues and events
affecting that science, industry and market from 2013 onwards:
Our report discusses issues and events
affecting that science, industry and market from 2013 onwards:
• Cancer research and treatment
• DNA hypomethylation in autoimmune diseases
• Neurodegenerative diseases
• Neurological disorders
• Understanding of epigenetic markers and mechanisms.
Also, you find coverage of these aspects of the field:
• Genomics, proteomics and metabolomics
• Translational research
• Licensing, outsourcing and academic collaborations
• Developments with stem cells
• Orphan indications and patient subpopulations
• Companion diagnostics and stratified medicine.
See what the future holds. You investigate technological, commercial, economic and political matters, with emphasis on companies, competition and business outlooks.
Leading companies and market value
Overall world revenue for epigenetic applications will reach $2,518m in 2013, our report forecasts. The R&D pipeline is diverse and promising. That industry holds great potential for further investment, development and growth.
I need to check the definition: what does "epigenetic therapies" mean... And the revenue of $2.5m looks not very serious, as well as the term "epigenetic therapies"!
Research and development - see trends and possibilities
What's happening in epigenetic R&D? You see trends and outlooks there:
• HDAC inhibitors
• DNMT inhibitors
• Treatments targeting other enzymes
• MiRNA therapy
• Diagnostics pipeline.
Our study also discusses these technologies, among others:
• New drug targets
• Biomarkers
• Bioinformatics
• Next generation sequencing.
Discover progress. You assess innovations affecting the industry's future - hear about developments and find their significance. Our work explains, exploring many issues.
What affects the application of epigenetics?
 Our report discusses issues and events
affecting that science, industry and market from 2013 onwards:
Our report discusses issues and events
affecting that science, industry and market from 2013 onwards:• Cancer research and treatment
• DNA hypomethylation in autoimmune diseases
• Neurodegenerative diseases
• Neurological disorders
• Understanding of epigenetic markers and mechanisms.
Also, you find coverage of these aspects of the field:
• Genomics, proteomics and metabolomics
• Translational research
• Licensing, outsourcing and academic collaborations
• Developments with stem cells
• Orphan indications and patient subpopulations
• Companion diagnostics and stratified medicine.
See what the future holds. You investigate technological, commercial, economic and political matters, with emphasis on companies, competition and business outlooks.
Leading companies and market value
Overall world revenue for epigenetic applications will reach $2,518m in 2013, our report forecasts. The R&D pipeline is diverse and promising. That industry holds great potential for further investment, development and growth.
I need to check the definition: what does "epigenetic therapies" mean... And the revenue of $2.5m looks not very serious, as well as the term "epigenetic therapies"!
Labels:
another magic cure,
Big Pharma,
bubble,
deconstruction,
failure,
future foresight,
quote of the day,
shit of the day
Shit of the day. Heineken as a critical resource
Labels:
deconstruction,
failure,
future foresight,
nice idea,
real science,
semantic,
shit of the day,
video
Thursday, February 21, 2013
Personalized medicine - impersonal BS.
Very interesting opinion in the article: The Op-Ed: Entering The Golden Age Of Big Data
Medicine is finally moving fully into the Information Age. Medical data is exploding and becoming increasingly digital. It is being aggregated and correlated in our Electronic Medical Records. All kinds of associations are waiting to be discovered in this mass of medical data we are suddenly aggregating.
And we are finally getting a chance to read our personal instruction manuals, i.e. our genomes, proteomes, communicomes, microbiomes, viromes… Doctors can now see a disease risk developing before patients even feel symptoms. And providers can use genetic data to custom tailor the right therapy, or even the right screening frequency.
 Imagine a future where your physician knows you’re sick before you do or where personalized, genetics-based decisions about the effectiveness of a drug are predetermined using microchip technology or your stored genome in your EMR. This future is much closer than most people think. We can now know which drug is going to work best on an individual, or which embryo is carrying a fatal mutation, or which individual is benignly carrying an infectious agent or recessive genetic defect.
Imagine a future where your physician knows you’re sick before you do or where personalized, genetics-based decisions about the effectiveness of a drug are predetermined using microchip technology or your stored genome in your EMR. This future is much closer than most people think. We can now know which drug is going to work best on an individual, or which embryo is carrying a fatal mutation, or which individual is benignly carrying an infectious agent or recessive genetic defect.
Well, the same mantra, the same intention to propaganda the main-stream. I do not know for what particular purpose but we have seen similar promises in connection with genome project etc. I think that this clever guy does not believe himself in what he is talking about. Otherwise he is not so clever...
And what about the following absolutely materialistic statement:
Medicine is becoming a Big Data problem. We are all just piles of carbon and oxygen and nitrogen. What makes us different is how those atoms are arranged and interact. This is Information. And it means therapeutic decisions must also be personalized, including the drugs to treat each individual’s unique illness and genome. Healthcare IT will lead us to this Golden Age.
I am not just piles of carbon and oxygen and nitrogen - I would like to be something more, not only in material field. I do not need this kind of Golden Age imagined here - let me back to miself-designed future without bogus of personalized medicine!
Labels:
bubble,
deconstruction,
evidence based medicine,
failure,
future foresight,
nice idea,
paradigm,
politics,
quantum,
real science,
shit of the day
Shit of the day. It could be true...

Labels:
chaos,
cognitive,
deconstruction,
future foresight,
politics,
shit of the day
Wednesday, February 20, 2013
Off-label anti-cancer drugs: 30%
 From here.
From here.
Overall, about 70 percent of the doses of the ten chemotherapies were used "on-label," which means the doses were used in line with FDA approval. The other 30 percent was used to treat cancers the drug regulators never approved.
Tuesday, February 19, 2013
Shit of the day. More fun foods for the masses!

Labels:
another magic cure,
chaos,
cognitive,
deconstruction,
future foresight,
masterpiece of the day,
nice idea,
paradigm,
politics,
real science,
semantic,
shit of the day
Monday, February 18, 2013
ABC of elites. Dugin
LOL!!! Very simply about very serious subject :)
Labels:
chaos,
cognitive,
deconstruction,
depression,
future foresight,
masterpiece of the day,
paradigm,
politics,
quantum,
real science,
semantic,
video
Music of the week. I can do anything!
Labels:
another magic cure,
chaos,
cognitive,
deconstruction,
future foresight,
magic,
masterpiece of the day,
music of the week,
nice idea,
politics,
quantum,
quote of the day,
real science,
semantic,
video
Shit of the country. Nemtsov
Labels:
cognitive,
deconstruction,
politics,
shit of the day,
video
Sunday, February 17, 2013
A.I.Fursov. ABC of informational society
Labels:
cognitive,
deconstruction,
future foresight,
nice idea,
paradigm,
politics,
video
Saturday, February 16, 2013
Targeted failure of the week. Post No 51-52. Xalkori, ABT-199 and any others
 Let's start with Xalkori
– I have already
written that ALK-mutation is the key to success of the
treatment... And what should do those who do not have this kind of
mutation? And... guess what? According
to the germans Xalkori does not work well even for those who do
hav this mutation!!!
Let's start with Xalkori
– I have already
written that ALK-mutation is the key to success of the
treatment... And what should do those who do not have this kind of
mutation? And... guess what? According
to the germans Xalkori does not work well even for those who do
hav this mutation!!!
Germany's
Institute for Quality and Efficiency in Health Care (IQWiG) said in a
preliminary benefit assessment that Xalkori crizotinib from Pfizer
Inc. (NYSE:PFE) provides "no additional benefit" over
docetaxel or pemetrexed-containing chemotherapy in patients with
previously treated, advanced non-small cell lung cancer (NSCLC) whose
tumors are anaplastic lymphoma kinase (ALK)-positive -- Xalkori's
approved indication. IQWiG said Xalkori may lead to improvements in
health-related quality of life vs. the comparators requested by
Germany's Federal Joint Committee (G-BA) in patients for whom
chemotherapy is still an option. However, IQWIG said patients
receiving Xalkori were at an increased risk for serious adverse
events.
Because
Pfizer's dossier did not include the requested data, IQWiG said
Xalkori also has "no additional benefit" over G-BA's
requested comparator of best supportive care (BSC) in patients for
whom chemotherapy is no longer an option.
And
the second failure is ABT-199,
and not only this very targeted drug but other several candidates! It
is a disaster for the company – to invest a huge amount of
resources and time and then fail...
AbbVie
Inc., the drug company that split off from Abbott Laboratories at the
start of the year, suspended five studies on its experimental
leukemia and lymphoma medicine after two patient deaths.
The
patients died from tumor lysis syndrome, said Tracy Sorrentino, a
spokeswoman for North Chicago, Illinois-based AbbVie. The
complication stems from the rapid destruction of malignant cells
after treatment that can trigger acute kidney failure. It occurs most
often with large tumors such as those found in leukemia and lymphoma
patients, according to the National Institutes of Health.
ABT-199
is one of the company’s most-promising new compounds and was slated
to start the final round of testing usually required for U.S.
regulatory approval this year.
“We
have every expectation that these trials will come off the partial
clinical hold and we’ll be able to initiate Phase 3 trials in 2013
as planned,” she said. “ABT-199 is a highly- potent agent and can
result in the tumors reducing really quickly,” she said. “We are
working to refine the dose.”
After the complication was discovered, AbbVie and its partner, Roche Holding AG, suspended the dose-escalation portion of the studies to determine the amount of drug that is safest and most effective, Sorrentino said. The risk stems from the drug’s potency and can be managed if the dose is carefully controlled, she said.
Labels:
Big Pharma,
bubble,
chaos,
deconstruction,
depression,
evidence based medicine,
failure,
future foresight,
nice idea,
real science,
shit of the day,
targeted failure
Masterpiece of the day. Fake?

Labels:
masterpiece of the day,
nice idea,
politics,
real science
Shit of the day. Europe does not have plan "B"
Healthy dose of conspirology from Pereslegin:
Labels:
another magic cure,
bubble,
chaos,
cognitive,
deconstruction,
depression,
failure,
future foresight,
paradigm,
politics,
real science,
shit of the day,
video
Friday, February 15, 2013
Masterpiece of the day. The Ocean
Labels:
another magic cure,
magic,
nice idea,
paradigm,
real science,
video
Shit of the day. Sochi 2014

Labels:
chaos,
cognitive,
depression,
failure,
politics,
shit of the day
Quote of the day. Failure.
Labels:
another magic cure,
cognitive,
deconstruction,
failure,
future foresight,
magic,
masterpiece of the day,
nice idea,
paradigm,
quantum,
quote of the day,
real science,
semantic,
targeted failure
Thursday, February 14, 2013
Shit of the day. When the russians come

Labels:
chaos,
cognitive,
failure,
future foresight,
nice idea,
paradigm,
Partisan of Death Valley,
politics,
question of the day,
real science,
shit of the day
Wednesday, February 13, 2013
Another magic cure against cancer. Post No 41. Modified virus code-named JX-594
And this time we have magic cure against liver cancer (probably HCC):
The virus used in the vaccine that helped eradicate smallpox is now working its magic on liver cancer. A genetically engineered version of the vaccinia virus has trebled the average survival time of people with a severe form of liver cancer, with only mild, flu-like side effects.
Two of the patients on the highest viral dose were still alive more than two years after the treatment. "It's a very substantial survival benefit," says Laurent Fischer, president of Jennerex, the company in San Francisco developing the treatment under the trade name Pexa-Vec.
Besides shrinking the primary tumour, the virus was able to spread to and shrink any secondary tumours outside the liver. "Some tumours disappeared completely, and most showed partial destruction on MRI scans," says David Kirn, head of the study at Jennerex. Moreover, the destruction was equally dramatic in the primary and secondary tumours.
Labels:
another magic cure,
bubble,
evidence based medicine,
future foresight,
nice idea,
real science
Masterpiece of the day. Gay-elite vs ...
Labels:
another magic cure,
cognitive,
deconstruction,
failure,
future foresight,
masterpiece of the day,
nice idea,
politics,
real science,
shit of the day
Quote of the day. The problem...

Labels:
another magic cure,
chaos,
cognitive,
deconstruction,
future foresight,
masterpiece of the day,
nice idea,
paradigm,
Partisan of Death Valley,
question of the day,
quote of the day,
real science,
semantic
Tuesday, February 12, 2013
New drugs in 2012. Business summary. Chemical structures. Fresh numbers.
And the
summary is here.
And the
numbers are huge!
New drugs
for cancer and rare diseases come with big price tags
DRUG COST
Gattex
$295,000/year
Kalydeco
$294,000/year
Juxtapid
$200,000–$300,000/year
Elelyso $150,000/year
Iclusig
$115,000/year
Zaltrap $11,000/month
Cometriq
$9,900/month
Kyprolis
$9,550/month
Stivarga
$9,350/month
Inlyta <
$8,900/month
Bosulif
$8,200/month
Erivedge
$7,500/month
Xtandi
$7,450/month
There is a
very old proverb: “It is better to be healthy and rich than sick and poor”. Undoubtedly, if you are sick – you will be
poor!
Labels:
another magic cure,
Big Pharma,
bubble,
deconstruction,
depression,
evidence based medicine,
future foresight,
numbers,
paradigm,
real science,
shit of the day
Targeted failure of the week. Post No 50. Romosozumab (AMG 785)
We have another targeted failure, and this time it is another mAb (basically mAbs fails pretty often :))
Amgen Inc. and partner UCB Group discontinued development of romosozumab (AMG 785) to improve fracture healing. The partners made the decision after top-line data showed the compound missed the primary endpoint of improving time to radiographic healing at 52 weeks vs. placebo in a Phase II trial to treat unilateral tibial diaphyseal fracture after fixation with an intramedullary nail. The partners said safety was not a factor. The humanized mAb against sclerostin has also completed a Phase II trial in patients with a unilateral hip fracture who have received surgical fixation, but data are not yet available. Amgen declined to provide details, but said its decision to discontinue development of romosozumab in fracture healing was also impacted by recent FDA guidance requiring positive data from two Phase III trials per fracture site to support approval of a fracture healing indication.
Labels:
Big Pharma,
bubble,
chaos,
deconstruction,
depression,
failure,
paradigm,
shit of the day,
targeted failure
Masterpiece of the day. Crisis of identity. A.I.Fursov
Labels:
another magic cure,
chaos,
cognitive,
deconstruction,
failure,
magic,
masterpiece of the day,
nice idea,
paradigm,
politics,
quantum,
real science,
video
Monday, February 11, 2013
Disaster in Novo Nordisk: Tresiba® (insulin degludec) - not approved by FDA!!!
It should be something very wrong with FDA or Tresiba or Novo Nordisk but the news is very serious:
In November, panel of federal health advisers recommended that the government approve degludec. The FDA is not required to follow the group’s advice, but it often does.
Tresiba contains long-acting insulin, and Ryzodeg contains both long- and short-acting insulins.
NEW YORK — Novo Nordisk AS says the Food and Drug Administration rejected its applications for approval of once-per-day insulin drugs to treat diabetes.
The Danish drugmaker says it received a “complete response letter” from the FDA regarding its applications for approval of Tresiba and Ryzodeg, or insulin degludec, on Friday. Such a letter indicates the agency determined the application cannot be approved in its current form.
Tresiba contains long-acting insulin, and Ryzodeg contains both long- and short-acting insulins.
Labels:
chaos,
cognitive,
depression,
failure,
shit of the day
Shit of the decade. Liberalism
Labels:
chaos,
cognitive,
deconstruction,
depression,
failure,
politics,
quantum,
quote of the day,
shit of the day
Welcome to Russian Olympic Games. Sochi 2014. Masterpiece of the year 2014
Labels:
cognitive,
deconstruction,
depression,
failure,
future foresight,
masterpiece of the day,
nice idea,
politics,
real science,
semantic,
shit of the day,
video
Sunday, February 10, 2013
Targeted failure of the week. Post No 49. FDA approved endovascular treatment
Very interesting news:
It's another case of a beautiful idea colliding with some ugly facts.
The beautiful idea is the notion that clearing the blocked artery of a stroke patient with a device snaked right up to the blockage would salvage threatened brain cells and prevent a lot of disability.
A lot of stroke patients in the U.S. are already getting this endovascular (within-the-artery) treatment because the Food and Drug Administration approved the devices without clinical proof that they work. Medicare has begun paying for the treatment.
But now come those ugly facts. Three studies have now found no difference in outcome between patients who got the endovascular treatment along with an intravenous dose of a clot-busting drug called tPA, or Alteplase, and other patients who got only tPA.
"There wasn't really clear evidence that endovascular therapy added to tPA ... was better overall than tPA as the standard treatment," Dr. Joseph Broderick told MedPage Today. "It was hard to detect a signal of benefit in the studies that were presented."
Well, the question becoms: how many FDA-approved treatments outthere are not effiient (at least if not harmful)? Some relevant information in one of my previous posts.
Quote of the day. Wrong direction???

Labels:
another magic cure,
chaos,
cognitive,
deconstruction,
failure,
masterpiece of the day,
nice idea,
paradigm,
quote of the day,
real science,
shit of the day
Masterpiece of the day. The art
Amazing video is here: http://fotofilmi.ru/video/34789cddd652a41/Syujetnyiy-fotoart
Labels:
another magic cure,
cognitive,
deconstruction,
magic,
masterpiece of the day,
nice idea,
video
Saturday, February 9, 2013
Friday, February 8, 2013
Music of my life. Kolas, Piesniary i .... Rodny Kut
Labels:
another magic cure,
cognitive,
deconstruction,
magic,
masterpiece of the day,
music of the week,
nice idea,
paradigm,
quantum,
real science,
semantic
Garbage in, garbage out. Crowdsourcing.
Very
interesting news:
LONDON, Feb 7 (Reuters) - Seven European
drugmakers are to pool their research efforts with academic scientists and
smaller companies in a new 196 million euros ($265 million) project designed to
find tomorrow's medicines.
The project, backed by the European Union,
is the latest example of the drugs industry exploring ways to share early-stage
research, effectively taking lessons from the kind of open innovation that gave
the world Linux software.
As part of the European Lead Factory
scheme, pharmaceutical companies will contribute at least 300,000 chemical
compounds from their in-house collections and a further 200,000 will be
developed jointly by academia and small firms.
The idea is to use crowdsourcing to
generate novel ideas for tackling certain diseases, which can then be tested by
screening compounds using shared pharmaceutical industry know-how.
"It's a big change for companies
because their compound libraries have usually been kept very secret," said
Ton Rijnders, scientific director of Dutch non-profit group TI Pharma, who is
helping to run the project.
"They are doing this because it is
cheaper than building ever larger libraries on their own - and partnering with
academics gives them access to innovative ideas."
The seven drugmakers involved in the scheme
are Bayer , AstraZeneca, Sanofi, Lundbeck , Merck KGaA, UCB and Janssen, the
European arm of Johnson & Johnson.
Academic partners include universities in
Germany, Britain, the Netherlands and Denmark.
The programme is supported by the EU-led
Innovative Medicines Initiative (IMI), which supports early-stage collaborative
research in conjunction with academia.
The IMI was set up five years ago in a bid
to re-establish Europe as the "pharmacy of the world" and close a
growing gap with United States and Asia on drug research.
For academic researchers, the scheme will
offer unprecedented access to industry chemical collections. Industry,
meanwhile, should benefit from having independent scientists taking a fresh
look at their assets.
Well, the
key words here are “300,000 chemical compounds from their in-house collections”
(yes - the garbage can be called as collections – it is not a new approach) and
“crowdsourcing”.
Labels:
another magic cure,
Big Pharma,
bubble,
chaos,
cognitive,
deconstruction,
failure,
future foresight,
HTS,
numbers,
paradigm,
shit of the day
Subscribe to:
Comments (Atom)