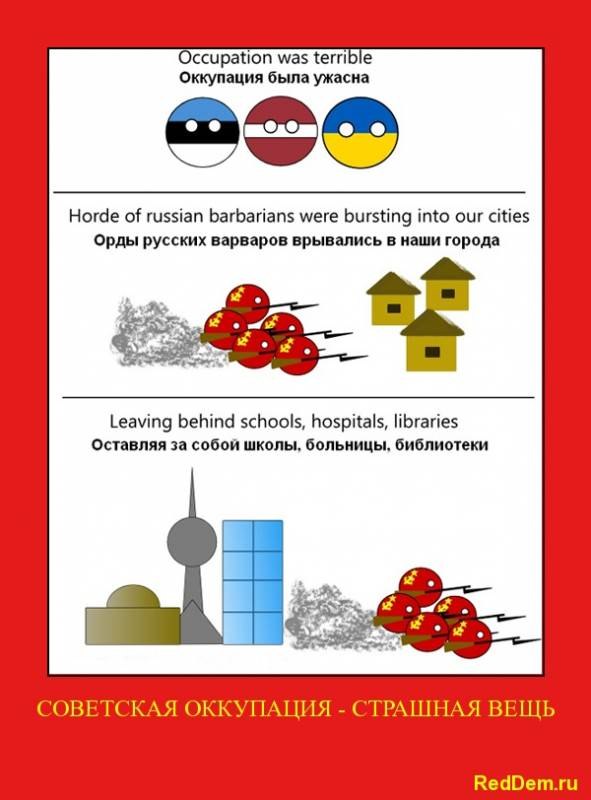
Sunday, March 31, 2013
Shit of the german agression on Cyprus

Labels:
chaos,
cognitive,
deconstruction,
depression,
nice idea,
shit of the day
Masterpiece of the day. One school in India.
I still believe in the prominent future of this epic country!


Saturday, March 30, 2013
Music of the week. Tsoj.
Labels:
cognitive,
deconstruction,
depression,
music of the week,
semantic,
video
Why gold is so cheap?
Why Isn’t Gold Higher? A very good question. The answer is here:
http://gonzalolira.blogspot.ru/2013/01/why-isnt-gold-higher.html
Enjoy it! Very rational analysis of the situation,
http://gonzalolira.blogspot.ru/2013/01/why-isnt-gold-higher.html
Enjoy it! Very rational analysis of the situation,
Labels:
bubble,
chaos,
deconstruction,
future foresight,
masterpiece of the day,
nice idea,
numbers
Shit of the day. Who are these people?

Cancer is almost cured!
Labels:
bubble,
cognitive,
deconstruction,
future foresight,
HTS,
paradigm,
shit of the day,
targeted failure,
video
Friday, March 29, 2013
China is ready
Labels:
deconstruction,
future foresight,
politics,
shit of the day,
video
Fake?

Labels:
another magic cure,
chaos,
deconstruction,
nice idea,
politics,
real science,
semantic,
shit of the day
Thursday, March 28, 2013
Shit of the day. Liberalism

Labels:
chaos,
deconstruction,
failure,
future foresight,
politics,
real science,
shit of the day
Wednesday, March 27, 2013
Masterpiece of the day. We win - you lose, sign here.

Labels:
chaos,
cognitive,
deconstruction,
depression,
future foresight,
macro economy,
masterpiece of the day,
nice idea,
paradigm,
Partisan of Death Valley,
politics,
real science,
semantic,
shit of the day
Somebody is very scared.
Labels:
deconstruction,
failure,
future foresight,
paradigm,
politics,
video
Tuesday, March 26, 2013
Shit of the currency devolvation

Labels:
deconstruction,
future foresight,
nice idea,
numbers,
politics,
real science,
shit of the day
Music of the week. Something stupid...
Masterpiece of understanding. Watson.
Labels:
another magic cure,
chaos,
cognitive,
Death Valley,
deconstruction,
evidence based medicine,
future foresight,
masterpiece of the day,
nice idea,
quote of the day,
real science,
semantic
Monday, March 25, 2013
Shit of the lie...

Labels:
chaos,
cognitive,
deconstruction,
failure,
nice idea,
politics,
real science,
shit of the day
Quote of the day
Лишь тот достоин жизни и свободы,
Кто каждый день за них идет на бой! И.В. Гёте
Кто каждый день за них идет на бой! И.В. Гёте
Sunday, March 24, 2013
Big Pharma for old men. Numbers
From here.
US biopharmaceutical companies are currently developing 465 new medicines
that target the 10 leading chronic conditions affecting people aged 65 and over,
according to new industry data.
The medicines, which are all now in clinical trials or under review by the
Food and Drug Administration (FDA), are diverse in scope, notes the
Pharmaceutical Research and Manufacturers of America (PhRMA), which has
published the data. The products include:
- 142 for diabetes, which affects 10.9 million Americans aged 65 and over -
around 26.9% of this age group, and with a total economic cost to the nation in
2007 of $174 billion;
 - 92 for rheumatoid arthritis and osteoarthritis (OA), which affect 1.3
million and 12.4 million Americans, respectively, in this age group.
Work-related OA costs the US up to $13.2 billion a year;
- 92 for rheumatoid arthritis and osteoarthritis (OA), which affect 1.3
million and 12.4 million Americans, respectively, in this age group.
Work-related OA costs the US up to $13.2 billion a year;
- 82 for Alzheimer's disease, which has an estimated 5.4 million US patients
and could afflict nearly eight million by 2030 unless a treatment or
preventative measure is found. Today, someone in the US develops the disease
every 68 seconds, and by 2050 this rate is expected to rise to one new case
every 33 seconds, creating up to 16 million patients by that time;
- 48 for heart failure, which affects 5.8 million US citizens, plus ischaemic
heart disease. For 2009, the total direct and indirect costs to the US of
cardiovascular disease and stroke was estimated to be $312.6 billion; and
-
40 for chronic obstruct pulmonary disease (COPD), which impacts more than 13
million adults in the US with the highest prevalence rate in people aged over
65. In 2010, the cost to the nation for COPD was put at $49.9 billion, including
$29.5 billion in direct health care expenditures, $8 billion in indirect
morbidity costs and $12.4 billion in indirect mortality costs.
PhRMA notes that the treatments which are currently in development include:
PhRMA notes that the treatments which are currently in development include:
- a medicine that aims to prevent or reverse progression of
Alzheimer's disease by using a human monoclonal antibody specifically designed
to draw beta amyloid protein away from the brain through the blood system;
- a medication that combines two long-acting drugs, allowing for once-daily
dosing in COPD;
- a potential first-in-class treatment for type 2 diabetes that increases
insulin secretion without causing insulin to significantly lower blood
usage;
- a product that recruits a patient's own neural stem cells to repair or
protect against damage to the central nervous system from stress hormones, which
can lead to depression; and
- a potential first-in-class medicine that
targets the pain associated with osteoarthritis by inhibiting a gene-encoding
protein that plays a role in inflammatory pain.
Among the targets for other medicines also currently in R&D for older people are:
- cataracts, which affect nearly 22 million Americans aged 40 and over. By age 80, more than half of all US citizens have cataracts, and the direct medical costs of treating them are put at $6.8 billion a year;
- chronic kidney disease, which is estimated to affect 13% of the US population, most of which are undiagnosed, and the prevalence for people aged 60 and older is put at 35%;
- depression, which affects more than 6.5 million older Americans, including 70% more women than men, and has become one of the nation's most expensive illnesses. Left untreated, depression costs more than $521 billion a year in absenteeism from work and lost productivity and $26 billion in direct treatment costs; and
- osteoporosis, which is responsible for two million broken US bones and $19 billion in related costs every year. By 2025, it is predicted that osteoporosis will be responsible for around three million fractures in the US and costs of $25.3 billion every year.
Among the targets for other medicines also currently in R&D for older people are:
- cataracts, which affect nearly 22 million Americans aged 40 and over. By age 80, more than half of all US citizens have cataracts, and the direct medical costs of treating them are put at $6.8 billion a year;
- chronic kidney disease, which is estimated to affect 13% of the US population, most of which are undiagnosed, and the prevalence for people aged 60 and older is put at 35%;
- depression, which affects more than 6.5 million older Americans, including 70% more women than men, and has become one of the nation's most expensive illnesses. Left untreated, depression costs more than $521 billion a year in absenteeism from work and lost productivity and $26 billion in direct treatment costs; and
- osteoporosis, which is responsible for two million broken US bones and $19 billion in related costs every year. By 2025, it is predicted that osteoporosis will be responsible for around three million fractures in the US and costs of $25.3 billion every year.
Labels:
Big Pharma,
bubble,
deconstruction,
future foresight,
nice idea,
numbers,
real science
Saturday, March 23, 2013
Targeted failure of the week. Post No 57. Cerulean's CRLX101
From here:
 Cerulean Pharma Inc. (Cambridge, Mass.) said CRLX101 given every other week plus best supportive care missed the primary endpoint of improving median overall survival (OS) vs. best supportive care alone in a Phase IIb trial to treat advanced non-small cell lung cancer (NSCLC). The open-label, Russian and Ukrainian trial enrolled 157 patients who progressed after one to two regimens of chemotherapy. Cerulean declined to disclose details. The company said it will focus on its other cancer programs for CRLX101 instead of continuing research in NSCLC.
Cerulean Pharma Inc. (Cambridge, Mass.) said CRLX101 given every other week plus best supportive care missed the primary endpoint of improving median overall survival (OS) vs. best supportive care alone in a Phase IIb trial to treat advanced non-small cell lung cancer (NSCLC). The open-label, Russian and Ukrainian trial enrolled 157 patients who progressed after one to two regimens of chemotherapy. Cerulean declined to disclose details. The company said it will focus on its other cancer programs for CRLX101 instead of continuing research in NSCLC.
And what is CLRX101? According to wikipedia (from 20130323):
CRLX101 is a novel approach to cancer chemotherapy that is currently under investigation in human trials, and is an example of a nanomedicine.
The agent represents a nanoparticle conjugate that consists of a drug delivery molecule, namely a cyclodextrin-based polymer (CDP) and an anti-cancer compound (camptothecin). It was developed by Dr. Mark E. Davis, professor of Chemical Engineering at the California Institute of Technology, and associates at Insert Therapeutics, Inc., now Calando Pharmaceuticals, Inc., hence the original name "IT-101". Its novel delivery mode allows the agent, and thus the toxic anti-cancer component, to be preferentially accumulated in cancer tissue. In turn, toxic side effect are expected to be reduced. The technology was licensed by Calando and Caltech to Cerulean Pharma Inc., in June, 2009.
Ok, nanomedicine fails as it was expected (I have written that nanomedicine sucks here) but it is interesting what was the rationale to use it - let's read t in wiki:
Rationale
 Camptothecin (CPT), an alkaloid extract with poor water solubility from plants such as camptotheca acuminata, exhibits anti-cancer activity possibly due, at least in part, by the inhibition of DNA topoisomerase I resulting in cell death. In CRLX101, CPT is linked covalently through a glycine link to the linear copolymer CDP, which in turn consists of alternating subunits of beta-cyclodextrin and polyethylene glycol (PEG). The CRLX101 nanoparticle is water soluble. After intravenous injection, active CPT is slowly released as the linkage is hydrolysed. The size of the nanoparticle (20-50 nm in diameter) facilitates its extravasation in the more leaky vessels of tumors via the enhanced permeability and retention effect and as a result, the anticancer drug is enhanced and retained in the tumor tissue.
Camptothecin (CPT), an alkaloid extract with poor water solubility from plants such as camptotheca acuminata, exhibits anti-cancer activity possibly due, at least in part, by the inhibition of DNA topoisomerase I resulting in cell death. In CRLX101, CPT is linked covalently through a glycine link to the linear copolymer CDP, which in turn consists of alternating subunits of beta-cyclodextrin and polyethylene glycol (PEG). The CRLX101 nanoparticle is water soluble. After intravenous injection, active CPT is slowly released as the linkage is hydrolysed. The size of the nanoparticle (20-50 nm in diameter) facilitates its extravasation in the more leaky vessels of tumors via the enhanced permeability and retention effect and as a result, the anticancer drug is enhanced and retained in the tumor tissue.
The rational - is a very old mantra which seems to e not rational anymore...
Labels:
bubble,
chaos,
deconstruction,
failure,
nanotechnology,
paradigm,
real science,
shit of the day,
targeted failure
Question of the day. What if?...

Labels:
chaos,
cognitive,
magic,
nice idea,
question of the day
Friday, March 22, 2013
Astra-Zeneca's way.
 AstraZeneca plc (LSE:AZN; NYSE:AZN) said at an investor day on Thursday that it is aiming to double its Phase III pipeline by 2016 and will focus small and large molecule R&D on three core areas: respiratory, inflammation and autoimmunity; cardiovascular and metabolic disease; and cancer. Pascal Soriot, who took over as CEO in October, said the pharma is aiming to create a portfolio with specialty care products to balance AstraZeneca's expertise in primary care.
AstraZeneca plc (LSE:AZN; NYSE:AZN) said at an investor day on Thursday that it is aiming to double its Phase III pipeline by 2016 and will focus small and large molecule R&D on three core areas: respiratory, inflammation and autoimmunity; cardiovascular and metabolic disease; and cancer. Pascal Soriot, who took over as CEO in October, said the pharma is aiming to create a portfolio with specialty care products to balance AstraZeneca's expertise in primary care.
Well, the strategy is very clear... But why just double? Go further!
Labels:
Big Pharma,
deconstruction,
future foresight,
nice idea,
numbers,
paradigm,
Partisan of Death Valley,
shit of the day
Music of the week. Just secret
Labels:
another magic cure,
chaos,
cognitive,
deconstruction,
magic,
masterpiece of the day,
nice idea,
paradigm,
real science,
semantic,
video
Thursday, March 21, 2013
Lung cancer: the reality

This year, an estimated 228,190 people are going to be diagnosed with some form of lung or bronchus cancer. I wish I could say that advancements have been swift in treating this disease, but abstinence and awareness as to the effects of cigarette smoking have been front and center in helping to reduce instances of lung cancer over the past two decades. Between 2005 and 2009, the incidence of lung cancer in men has decreased by an average of 1.9% annually. For women, this trend only recently began to reverse, with diagnoses falling by just 0.3% annually between 2005 and 2009.
Labels:
deconstruction,
depression,
evidence based medicine,
failure,
future foresight,
numbers,
real science,
shit of the day,
targeted failure
Cancer facts and figures: 2013.
Link to pdf: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf
USA only however the trends are the same worldwide.
USA only however the trends are the same worldwide.
Music of the week. Very cognitive BG
Labels:
chaos,
cognitive,
depression,
music of the week,
semantic,
video
Wednesday, March 20, 2013
Masterpiece of the victory.

Labels:
cognitive,
deconstruction,
masterpiece of the day,
nice idea,
paradigm,
politics,
real science
Tuesday, March 19, 2013
Slava? Zaspokojtysia!
Labels:
deconstruction,
failure,
paradigm,
politics,
shit of the day,
video
The crisis. ABC.
In plain words...
Video: http://www.youtube.com/watch?v=z-VGTLp1J4w&feature=player_embedded
Video: http://www.youtube.com/watch?v=z-VGTLp1J4w&feature=player_embedded
Labels:
another magic cure,
black swan,
bubble,
cognitive,
deconstruction,
depression,
future foresight,
investment,
macro economy,
paradigm,
politics,
real science,
video
More than 900 Biologics in Development??? Good Luck!
 America's biopharmaceutical companies are using biological processes to
develop 907 medicines and vaccines targeting more than 100 diseases, according
to a new report (PDF) released today by the Pharmaceutical Research
and Manufacturers of America (PhRMA).
America's biopharmaceutical companies are using biological processes to
develop 907 medicines and vaccines targeting more than 100 diseases, according
to a new report (PDF) released today by the Pharmaceutical Research
and Manufacturers of America (PhRMA).
The report includes biologics in human clinical trials or under review by FDA
such as 338 cancer therapeutics that target several different types of solid
tumors, leukemia and lymphoma; 134 vaccines for infectious diseases; 71
medicines for autoimmune diseases including lupus, multiple sclerosis, and
rheumatoid arthritis; and 58 treatments for cardiovascular disease.
Among the biologic medicines in development are a genetically-modified
virus-based vaccine to treat melanoma; a monoclonal antibody for the treatment
of asthma; an antisense therapy for the treatment of leukemia; a recombinant
fusion protein to treat Type II diabetes.
Labels:
another magic cure,
Big Pharma,
bubble,
deconstruction,
evidence based medicine,
grey swan,
nice idea,
numbers,
paradigm,
Partisan of Death Valley,
politics,
real science,
shit of the day,
targeted failure
Nationalization of Pfizer's IP in India.
From here:
 India has essentially created a protectionist regime that harms US job creators.
India has essentially created a protectionist regime that harms US job creators.
"Despite being a member of the WTO and an important global trading partner, India has systematically failed to interpret and apply its IP laws in a manner consistent with recognised global standards," he said, adding that the Global IP Centre's International IP Index ranked India last in terms of overall IP protection.
Stating that in September last year, India revoked Pfizer's patent for a cancer medication, SUTENT, Waldron said this will now allow Indian generic companies to manufacture and sell generic copies of SUTENT long before the patent is set to expire.
 India has essentially created a protectionist regime that harms US job creators.
India has essentially created a protectionist regime that harms US job creators."Despite being a member of the WTO and an important global trading partner, India has systematically failed to interpret and apply its IP laws in a manner consistent with recognised global standards," he said, adding that the Global IP Centre's International IP Index ranked India last in terms of overall IP protection.
Stating that in September last year, India revoked Pfizer's patent for a cancer medication, SUTENT, Waldron said this will now allow Indian generic companies to manufacture and sell generic copies of SUTENT long before the patent is set to expire.
Why not? It is very simple solution for a poor country like India. And I think that this approach will be popular in the future for other communist not-so-rich countries who will try to take care about the health of people.
Labels:
Big Pharma,
chaos,
cognitive,
deconstruction,
failure,
future foresight,
macro economy,
nice idea,
paradigm,
politics,
real science
Quote of the day. 20 steps' rule

Labels:
chaos,
cognitive,
deconstruction,
future foresight,
nice idea,
numbers,
paradigm,
quote of the day,
real science,
semantic
Masterpiece of the day. He knew it...
Putin expected the nationalization on Cypros
Video is here:
http://www.youtube.com/watch?feature=player_embedded&v=P3Y9T9P0Vls
Video is here:
http://www.youtube.com/watch?feature=player_embedded&v=P3Y9T9P0Vls
Labels:
chaos,
cognitive,
masterpiece of the day,
nice idea,
politics,
real science,
shit of the day,
video
The drug song
Monday, March 18, 2013
Drug commercial
Labels:
another magic cure,
Big Pharma,
cognitive,
deconstruction,
nice idea,
real science,
shit of the day
Sunday, March 17, 2013
Music of the week. Illusionen
Masterpiece of the politics. PR failure of Medvedev
Labels:
chaos,
deconstruction,
failure,
masterpiece of the day,
nice idea,
paradigm,
politics,
real science,
shit of the day,
targeted failure,
video
:) Difference in mentality

Labels:
cognitive,
deconstruction,
masterpiece of the day,
nice idea,
paradigm,
politics,
real science
Quote of the day. Poesi
Labels:
another magic cure,
cognitive,
magic,
masterpiece of the day,
paradigm,
politics,
quote of the day,
video
Saturday, March 16, 2013
Another magic cure against cancer. Post No 42. LDK378 treats non-small cell lung cancer
This time is serious:
 The U.S. Food and Drug Administration has designated a compound developed by NovartisAG to treat a type of non-small cell lung cancer for fast-track development and review, the Swiss drugmaker said on Friday.
The U.S. Food and Drug Administration has designated a compound developed by NovartisAG to treat a type of non-small cell lung cancer for fast-track development and review, the Swiss drugmaker said on Friday.
 The U.S. Food and Drug Administration has designated a compound developed by NovartisAG to treat a type of non-small cell lung cancer for fast-track development and review, the Swiss drugmaker said on Friday.
The U.S. Food and Drug Administration has designated a compound developed by NovartisAG to treat a type of non-small cell lung cancer for fast-track development and review, the Swiss drugmaker said on Friday.
Novartis said the FDA had given "breakthrough therapy" designation to its LDK378 compound, a process aimed at speeding up the review of drugs that treat life-threatening conditions if the therapy has demonstrated efficacy.
LDK378 - seen as a potential future blockbuster - is designed to treat anaplastic lymphoma kinase positive (ALK+) metastatic non-small cell lung cancer (NSCLC). Sufferers tend to be non-smokers and younger than other lung cancer patients.
Novartis said two Phase II trials were under way and it planned to launch several Phase III trials later this year with first regulatory filing expected by early next year.
"This breakthrough therapy designation will allow us to collaborate more closely with the FDA and potentially to expedite the availability of an important new treatment option for patients with ALK+ NSCLC," said Alessandro Riva, Novartis head of oncology development
Labels:
another magic cure,
Big Pharma,
future foresight,
real science
Masterpiece of the crisis. This time - USA
Labels:
another magic cure,
chaos,
cognitive,
deconstruction,
depression,
evidence based medicine,
failure,
future foresight,
macro economy,
masterpiece of the day,
nice idea,
paradigm,
politics,
shit of the day
Friday, March 15, 2013
Drug Discovery Outsourcing: World Market 2013-2023
From an advertisement. $16.6bn - very impressive market! And this is only outsourcing... Just imagine the spending of Big Pharma for the same purposes!

Quintiles
Charles River Laboratories
Covance
Evotec
PPD.
Albany Molecular Research
Aptuit
Cyprotex
Domainex
Galapagos
GenScript
WIL Research Laboratories
WuXi AppTec.
Leading companies and market value in 2015

Overall world revenue for that market area
will reach $16.6bn in 2015, our work forecasts. We predict strong revenue
growth from 2012 to 2023.
Our study gives you analyses of these CROs:
Quintiles
Charles River Laboratories
Covance
Evotec
PPD.
We also give profiles of these service vendors, and discuss other
companies too:
Albany Molecular Research
Aptuit
Cyprotex
Domainex
Galapagos
GenScript
WIL Research Laboratories
WuXi AppTec.
Labels:
Big Pharma,
deconstruction,
future foresight,
nice idea,
numbers,
paradigm,
real science
Inventing disease and pushing pills
Elizabeth Loder: Inventing disease and pushing pills
From here.
The recent 2013 Selling Sickness conference in Washington, DC was chock full of fascinating speakers. In an earlier blog I discussed my participation in a panel discussion at the conference, and Rachel Hendrick has also blogged about the meeting. It was difficult to choose from the topics on offer during the two day gathering, and many of the most enticing talks seemed to be scheduled concurrently with others that were equally intriguing. Since there were so many speakers and topics at the meeting who deserve attention, here are some selected highlights from my notes with links to more information about their work:
Jeremy Gruber of the Council for Responsible Genetics spoke about the large amount of money being poured into research on genetics: “What the world of genomics doesn’t want you to know is that the vast majority of morbidity and premature mortality come from smoking, overeating, and lack of exercise. For research in behavioral and social sciences, where even a small improvement in our ability to alter behaviour could yield significant benefits, funding is low.”Rachel Liebert took a provocative look at what she terms “psychic policing,” surveillance, and disease mongering in public education. She noted that in the wake of the recent Connecticut school shootings the popular Dr Oz called for “a homeland security approach to mental illness,” voicing the popular but unproved assumption that most school shootings are carried out by the mentally ill and that “the proper response is medical.” According to Ms Liebert, the classroom is “at risk of becoming a psychiatric assessment unit” where teachers and others are on constant high alert for any unusual behavior that might indicate a budding psychiatric problem. Ms Liebert provided examples of commercial companies that have developed costly programmes to mitigate perceived risks and improve school safety. She suggested this might be considered disease mongering since it fosters discussion about psychiatric illness rather than access to guns. She refers to this as the “intersection of medicalisation and securitisation.”Making fun of the concept of “pre-disease” and its contribution to medicalisation, a psychiatrist in the audience stood up and said “My diagnosis is pre-psychosis, but I’ve warded it off by wealth, education, an intact family, and living in safe neighborhoods. I have connections in my church. The treatment has worked marvelously and I’ve avoided hospitalisation. I want to thank the genome project for adding thousands of papers that show it’s nurture not nature that determines we will be healthy…we automatically assume [a drug] is the treatment… but really it’s the matrix in which the gene exists. It’s the environment.”Rhetorician Judy Segal of the University of British Columbia compared old advertisements for patent medicines with the pharmaceutical advertisements of today. Depressingly little has changed. “Drug marketing is one way consumers learn what their symptoms might add up to,” said Dr Segal. “Patent medicine ads hailed an audience that was tired, nervous, constipated, and broadly disappointed. Drug ads today are much the same…We are broadly vulnerable and at least a little sick. We are at our computers with better resources, but with many of the same concerns. Lydia Pinkham’s vegetable compound helped to constitute the female subject now considering Yaz for moodiness, Premarin for a menopausal vagina, Gardasil for her daughter, and Zoloft for depression.”Finally, for pure fun I advise you to watch the following list of videos. These are just some of those shown at the “Selling Sickness Popcorn Showcase” on the first evening of the conference. The first on the list is my favorite. Enjoy!
Labels:
another magic cure,
Big Pharma,
chaos,
deconstruction,
evidence based medicine,
failure,
future foresigh,
nice idea,
paradigm,
real science,
shit of the day
Alzheimer's Disease. FDA changes the rules?
From here:
The Food and Drug Administration plans to loosen the rules for approving new treatments for Alzheimer’s disease.
 Drugs in clinical trial would qualify for approval if people at very early stages of the disease subtly improved their performance on memory or reasoning tests, even before they developed any obvious impairments. Companies would not have to show that the drugs improved daily, real-world functioning.
Drugs in clinical trial would qualify for approval if people at very early stages of the disease subtly improved their performance on memory or reasoning tests, even before they developed any obvious impairments. Companies would not have to show that the drugs improved daily, real-world functioning.
For more than a decade, the only way to get Alzheimer’s drugs to market was with studies showing that they improved the ability of patients not only to think and remember, but also to function day to day at activities like feeding, dressing or bathing themselves.
The proposed policy could also be a boon for the pharmaceutical industry and researchers. They have often felt stymied by regulations that left them uncertain of how to get drugs tested and approved for marketing to people early in the course of Alzheimer’s, when the medications are most likely to be useful.
Wait a minute... What about "evidence based approach"? Well... Who cares! If there is a market for AD Big Pharma has to explore it! Who else would do it? And FDA just helps here in the way it can help...
Labels:
another magic cure,
Big Pharma,
bubble,
deconstruction,
evidence based medicine,
future foresight,
nice idea,
paradigm,
real science,
shit of the day
Thursday, March 14, 2013
Music of the week. Nothing else matters...
Labels:
cognitive,
depression,
magic,
music of the week,
real science,
video
Shit of the day. Stability
Labels:
cognitive,
deconstruction,
depression,
failure,
future foresight,
nice idea,
shit of the day
Wednesday, March 13, 2013
Big Pharma avoids Big Taxes.
Big Pharma avoids Big Taxes. Big Pharma has big experience and knowledge how to save money!
For years, multinationals such as Pfizer Inc., Merck & Co. and Johnson
& Johnson have been moving ownership of patents and trademarks to
subsidiaries in low- or no-tax countries. This has allowed drug companies, as
well as businesses in several other industries, to skirt paying U.S. taxes on
sales of those products unless the money is returned home.
While the practice of shifting assets and profits overseas is legal, that
could change. As the trend continues to grow in an era when the government is
desperate to raise revenue, the strategy has drawn the ire of legislators eager
to shut it down.
“The right kind of tax reform could do a lot to bring corporate profits back
to the United States for investment and job creation,” said Charles Grassley, a
U.S. senator from Iowa, in an e-mail. “The current system provides an incentive
for companies to keep money overseas indefinitely.”
Merck and J&J were the biggest drug company winners in 2012 with savings
of about $2 billion each attributable to the strategy, according to regulatory
filings. The reports by the six drugmakers, filed last month, come as U.S.
lawmakers are debating potential tax code changes designed to shrink the federal
budget deficit and crank up job-producing business activity in the U.S.
Labels:
Big Pharma,
cognitive,
deconstruction,
evidence based medicine,
future foresight,
investment,
macro economy,
nice idea,
paradigm,
politics,
quantum,
real science
Physicians? Corrupted!
I have written that physicians IN GENERAL are not interested in development of innovative products, this segment of health care industry is corrupted as anything else in this industry. And here is an example:
In Bergen and Passaic counties, 38 doctors and two hospitals received more than $25,000 from pharmaceutical companies – making a combined $4.5 million, according to the report by ProPublica, a non-profit investigative news organization that compiled the figures from information major drug companies filed with the federal government.
While doctors are often paid to speak about drugs they have used on patients and believe in, the practice has raised persistent ethical concerns. Drug makers say they are tapping into the medical expertise of physicians to teach their peers or conduct research, but others worry the practice may unduly influence the medications prescribed to patients.
Labels:
Big Pharma,
bubble,
deconstruction,
depression,
future foresight,
macro economy,
numbers,
paradigm,
Partisan of Death Valley,
politics,
shit of the day
Targeted failure of the week. Post No 56. Quizartinib
The molecule is too long? Or too complicated? In any way something went wrong with the molecule!
Eyebrows have been raised after Astellas Pharma decided to end its
collaboration with Ambit Biosciences Corp to develop the latter's acute myeloid
leukaemia treatment quizartinib.
Back in 2009, the companies entered into a collaboration focusing on FMS-like
tyrosine kinase-3 (FLT-3) inhibitors, including quizartinib. Now, the Japanese
drugmaker has exercised its right to terminate the agreement, citing "strategic
reasons", effective September 3, 2013.
Tuesday, March 12, 2013
A.I.Fursov. Analysis of 2012.
Labels:
another magic cure,
chaos,
cognitive,
deconstruction,
future foresight,
nice idea,
numbers,
paradigm,
Partisan of Death Valley,
politics,
real science,
video
Targeted Failure of the week. Post No 55. Perifosine
From here and here.
 Aeterna Zentaris Inc. (NASDAQ: AEZS) (TSX: AEZ) (the "Company") today announced that an independent Data Safety Monitoring Board ("DSMB") has recommended discontinuing the ongoing Phase 3 study comparing the efficacy and safety of perifosine to placebo when combined with bortezomib (Velcade®) and dexamethasone in patients with relapsed or relapsed/refractory multiple myeloma. Based on the outcome of its pre-planned interim analysis of efficacy and safety, the DSMB recommended that patient enrollment be stopped and the study discontinued. The DSMB reported that it was highly unlikely the study would achieve a significant difference in its primary endpoint, progression free survival; no safety concerns were raised.
Aeterna Zentaris Inc. (NASDAQ: AEZS) (TSX: AEZ) (the "Company") today announced that an independent Data Safety Monitoring Board ("DSMB") has recommended discontinuing the ongoing Phase 3 study comparing the efficacy and safety of perifosine to placebo when combined with bortezomib (Velcade®) and dexamethasone in patients with relapsed or relapsed/refractory multiple myeloma. Based on the outcome of its pre-planned interim analysis of efficacy and safety, the DSMB recommended that patient enrollment be stopped and the study discontinued. The DSMB reported that it was highly unlikely the study would achieve a significant difference in its primary endpoint, progression free survival; no safety concerns were raised.
Just one comment: despite the fact that perifosine is called as a targeted compound, in the reality it is not - it is involved in a range of mechanisms without exhibiting any specific targeting properties:
Labels:
deconstruction,
failure,
HTS,
real science,
shit of the day,
targeted failure
Pereslegin. New inspiration... New ideas... New understanding
Labels:
another magic cure,
cognitive,
deconstruction,
future foresight,
magic,
nice idea,
paradigm,
Partisan of Death Valley,
quantum,
quote of the day,
real science,
semantic,
video
Shit of the day. The respect of post-modern.
Just show to everybody outthere that you do not care to show your respect to people you encounter!
Labels:
chaos,
deconstruction,
failure,
shit of the day,
video
Monday, March 11, 2013
Masterpiece of the day. Spring.
Labels:
chaos,
cognitive,
deconstruction,
future foresight,
masterpiece of the day,
music of the week,
nice idea,
paradigm,
Partisan of Death Valley,
politics,
real science,
semantic,
video
Shit of the situation... Unexpectedly we bacome tricked...

Labels:
chaos,
cognitive,
deconstruction,
depression,
failure,
future foresight,
nice idea,
politics,
real science,
shit of the day
Subscribe to:
Comments (Atom)