Friday, December 26, 2014
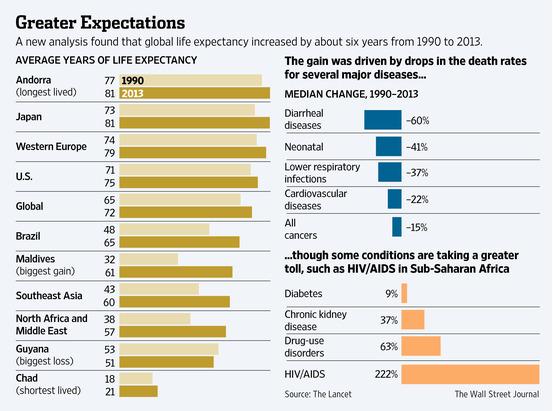
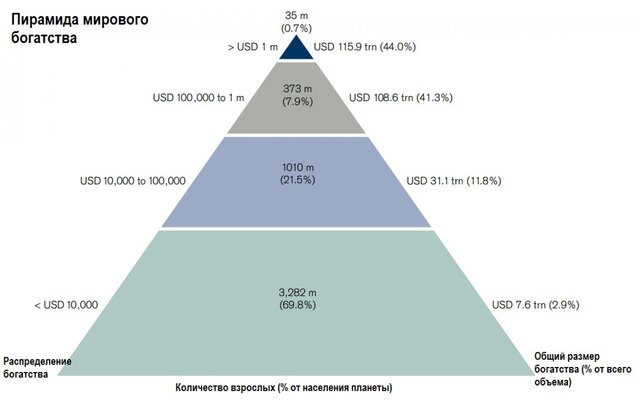
Fake?

Labels:
another magic cure,
cognitive,
deconstruction,
investment,
masterpiece of the day,
nice idea,
real science
Thursday, December 25, 2014
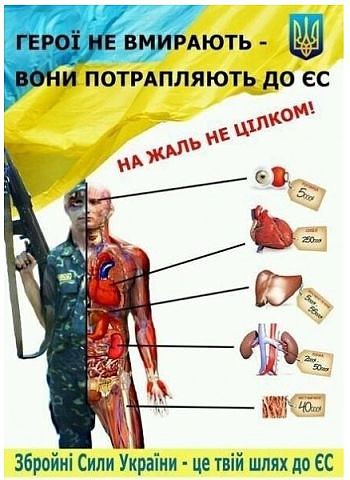
Ukraine will be free!
Labels:
another magic cure,
Big Pharma,
Death Valley,
deconstruction,
depression,
evidence based medicine,
failure,
investment,
numbers,
paradigm,
politics,
real science,
shit of the day
Just liver cancer
Labels:
deconstruction,
depression,
real science,
video
Tuesday, December 23, 2014
Monday, December 22, 2014

Labels:
another magic cure,
cognitive,
deconstruction,
masterpiece of the day,
nice idea,
shit of the day
Music of the week...
Labels:
cognitive,
deconstruction,
depression,
music of the week,
paradigm,
real science,
video
Saturday, December 20, 2014
Patent losses 2015.
Labels:
Big Pharma,
bubble,
deconstruction,
depression,
future foresight,
real science
Let's live forever!!
Labels:
Big Pharma,
deconstruction,
future foresight,
nice idea,
numbers,
real science
Fake?

Labels:
chaos,
cognitive,
deconstruction,
future foresight,
nice idea,
paradigm,
politics,
real science
Friday, December 19, 2014
21st century...

Labels:
bubble,
cognitive,
deconstruction,
depression,
failure,
masterpiece of the day,
nice idea,
paradigm,
shit of the day
Wednesday, December 17, 2014
Tuesday, December 16, 2014
Saturday, December 13, 2014
Nexavar vs communists...
http://www.biocentury.com/dailynews/company/2014-12-12/bayer-fails-to-block-generic-nexavar-in-india
 The Supreme Court of India dismissed an appeal by Bayer AG (Xetra:BAYN) and upheld a compulsory license issued to Natco Pharma Ltd. (BSE:NATCO; NSE:NATCOPHARM) to manufacture and market a generic version of cancer drug Nexavar sorafenib. Bayer had filed a Special Leave Petition seeking to overturn a Mumbai High Court decision that also left the license in place.
The Supreme Court of India dismissed an appeal by Bayer AG (Xetra:BAYN) and upheld a compulsory license issued to Natco Pharma Ltd. (BSE:NATCO; NSE:NATCOPHARM) to manufacture and market a generic version of cancer drug Nexavar sorafenib. Bayer had filed a Special Leave Petition seeking to overturn a Mumbai High Court decision that also left the license in place.
The Indian Patent Office granted Natco India's first compulsory license in March 2012 after finding Bayer did not make Nexavar available to the public at a "reasonably affordable price" (see BioCentury Extra, March 12, 2012).
The license allows Natco to sell its drug in India prior to the 2021 expiration of Bayer's local patent. The Indian company is permitted to sell its version at a price not exceeding Rs8,800 ($141.68) for a month's supply of 120 tablets. Bayer markets Nexavar at Rs280,428 ($4,515).
A Bayer spokesperson said the company is "analyzing the order and will determine any future course of action afterwards."
Bayer and the Onyx Pharmaceuticals Inc. subsidiary of Amgen Inc. (NASDAQ:AMGN) have a worldwide co-development agreement for Nexavar outside of Japan, where Bayer owns rights. Nexavar, an inhibitor of
CRAF (RAF1) and multiple receptor tyrosine kinases, is approved in more than 100 countries to treat kidney, liver and thyroid cancers.
Labels:
another magic cure,
Big Pharma,
deconstruction,
failure,
masterpiece of the day,
nice idea,
politics,
real science
Wednesday, December 10, 2014

Labels:
another magic cure,
cognitive,
deconstruction,
evidence based medicine,
masterpiece of the day,
paradigm,
real science
Saturday, December 6, 2014
The oil

Labels:
bubble,
chaos,
deconstruction,
nice idea,
politics,
real science,
shit of the day
Thursday, December 4, 2014
We win - you lose, sign here...

Labels:
deconstruction,
depression,
nice idea,
numbers,
paradigm,
real science
Wednesday, December 3, 2014

Labels:
another magic cure,
cognitive,
deconstruction,
masterpiece of the day,
nice idea,
quote of the day,
real science
Friday, November 28, 2014
Fake?

Labels:
chaos,
cognitive,
deconstruction,
future foresight,
masterpiece of the day,
nice idea,
politics
Thursday, November 27, 2014
Wednesday, November 26, 2014
Fake?

Labels:
chaos,
cognitive,
deconstruction,
future foresight,
nice idea,
paradigm,
politics,
real science,
shit of the day
Why not?

Labels:
cognitive,
deconstruction,
future foresight,
nice idea,
politics,
real science
Tuesday, November 25, 2014
The higher failure rate...
While the average time it takes to bring a drug through clinical trials has grown shorter in recent years, the rate of success of those drugs has gone down by almost half, to just 12 percent.
Almost all the attention from Tuesday's release by the Tufts Center for the Study of Drug Development focused on the eye-popping $2.6 billion cost it cited as needed to develop a new drug.
"The higher failure rate had a substantial impact on R&D costs. Higher out-of-pocket costs of conducting R&D, and proportionally more failures in clinical testing, are what really drove the increase in costs per approved drug," DiMasi said during a presentation Tuesday.
Based on an analysis of 1,442 experimental drugs that were in clinical tests in recent years through the end of 2013, DiMasi said the overall chance that a drug entering clinical development will be approved for marketing is just under 12 percent. That's down from 11 years ago, when the study set a success rate for drugs that enter human trials of 21.5 percent.
"Approximately seven out of eight compounds that enter the clinical testing pipeline will fail in development," he said. "Put another way, you need to put an average of 8.5 compounds in clinical development to get one approval."
DiMasi arrived at that figure using a weighted average, since as of the study, just 7 percent of the 1,442 drugs had actually been approved. Fully 80 percent had been abandoned by the companies developing them, and the other 13 percent were still in active development. DiMasi said it's likely that many of the drugs in later development will eventually earn approval, hence the overall 12 percent rate.
The chances of a drug moving from Phase 2 to Phase 3 testing was found to be 36 percent, while the rate at which a drug that finished Phase 3 tests went on to have an application for approval was 62 percent.
Another fact DiMasi said is increasingly relevant to the startup-heavy Massachusetts biotech scene: Small biotechs tend to push drugs from early into late-stage development more frequently than large companies, only to have them fail in later stages.
"There's reason to believe that small biotech firms will tend to keep their drugs in development longer than larger firms might, because, they are single or two-product firms, and if the product fails, the company goes under," he said.
http://www.bizjournals.com/boston/blog/bioflash/2014/11/tufts-study-it-takes-eight-drugs-in-clinical.html?page=all
http://www.bizjournals.com/boston/blog/bioflash/2014/11/tufts-study-it-takes-eight-drugs-in-clinical.html?page=all
Labels:
Big Pharma,
bubble,
Death Valley,
deconstruction,
failure,
numbers,
paradigm,
Partisan of Death Valley,
shit of the day
Monday, November 24, 2014
Sunday, November 23, 2014
15 jahren Putin. I win - you lose, sign here...

Labels:
black swan,
bubble,
cognitive,
deconstruction,
evidence based medicine,
future foresight,
macro economy,
masterpiece of the day,
nice idea,
numbers,
politics,
real science
Global pharma spending 'to soar 30% by 2018' - when health is a critical resource...
The world pharma market will
increase at a compound average growth rate (CAGR) of 4%-7% during 2013-18,
rising $305-$335 billion over the period (based on constant exchange rates)
compared with $194 billion growth seen in the previous five-year period,
2009-13, when the CAGR was 5.2%, says the research, from the IMS Institute for
Healthcare Informatics.
This fast growth will be
driven primarily by increased specialty drug innovation, greater access to
medicines and reduced impact of patent expiries, says the study. It also
forecasts that annual spending growth will spike this year at $70 billion, up
from $44 billion in 2013 and $26 billion in 2012 and will moderate in the years
to 2018, although remaining at higher levels than those seen during 2009-13.
“The higher level of spending
growth we’re projecting over the next five years reflects an unusual
combination of higher spending on the surge of innovative medicines for
patients and lower savings from patent expiries,” says Murray Aitken, IMH
Health senior vice president and executive director of the IMS Institute.
“This is particularly evident
this year and next in developing countries, and especially in the US, which
accounts for more than a third of the global market,” he adds.
The developed markets – led by
the US, the five major European markets (France, Germany, Spain, UK and Italy)
and Japan – will be the primary drivers of this increased growth; while they
will moderate as cost-containment measures further limit price levels, rising
volumes will continue to contribute to overall market growth. Only France and
Spain will see a contraction in pharmaceutical spend per capita in 2018, as a
result of policies aimed at curbing spending growth.
China, which is already the
world’s second-largest pharmaceutical market, will reach spending levels of
$155-$185 billion in 2018, the report adds.
Specialty medicines are
expected to contribute 40% of total global spending growth to 2018, with higher
expenditures particularly in the developed markets. Much of this growth will be
from products which bring patients new treatment options, with particularly
notable advances expected in the areas of oncology, autoimmune, respiratory,
antiviral and immunosuppressant therapies.
The recent surge in cancer
drug innovation will continue and contribute to global spending on all oncology
drugs, which is set to rise from $65 billion in 2013 to around $100 billion in
2018, while the introduction and uptake of potent new hepatitis C therapies are
forecast to producing spending totalling about $100 billion in the five years
to 2018, the report forecasts.
Moreover, almost 200 new drugs
are expected to be launched in the next five years. Over 2,000 products are
currently in late-stage development, a quarter of which are oncology drugs.
However, the IMS Institute notes that the availability of new medicines to patients
worldwide varies significantly by country and disease area and that, on
average, fewer than half the medicines launched during the previous five years
are now available across the major developed markets.
- The IMS Institute points out
that its estimates are measured at ex-manufacturer level and do not factor in a
range of rebates, discounts, taxes and other adjustments that affect the net
amount received by manufacturers. The impact of these factors is estimated to
reduce growth by $60-$80 billion, or around 25% of the increase forecast, over
the next five years, it says
Labels:
Big Pharma,
bubble,
deconstruction,
evidence based medicine,
macro economy,
nice idea,
numbers,
real science,
shit of the day
Friday, November 21, 2014

Labels:
chaos,
cognitive,
deconstruction,
failure,
masterpiece of the day,
nice idea
Wednesday, November 19, 2014
"Let's dance!" as a critical resource!
Labels:
another magic cure,
chaos,
cognitive,
deconstruction,
evidence based medicine,
nice idea,
real science,
video
Sexual health as a critical resource
Labels:
another magic cure,
bubble,
chaos,
cognitive,
evidence based medicine,
masterpiece of the day,
nice idea,
real science,
video
Monday, November 17, 2014
No money - no children...
Personally I do not have any comments...
Pfizer, the Bill & Melinda
Gates Foundation and the Children’s Investment Fund Foundation have teamed up
to expand access to the drug giant’s injectable contraceptive Sayana Press
(medroxyprogesterone acetate) in 69 of the world’s poorest countries.
Sayana Press combines a long-acting, reversible, contraceptive with an
all-in-one pre-filled, single-use, non-reusable Uniject injection system that
eliminates the need to prepare a needle and syringe. This allows the the drug
to be administered by health workers to women at home or in other convenient
settings.
“Pfizer saw an opportunity to
address the needs of women living in hard-to-reach areas, and specifically
enhanced the product’s technology with public health in mind,” said John Young,
president of Pfizer's global established pharma business.
More than 200 million women in
developing countries want to delay pregnancy or prevent undesired pregnancy but
are not using any method of contraception. “Far too many women die or are
harmed because of unwanted pregnancies,” said Michael Anderson, chief executive
at the Children’s Investment Fund Foundation, noting that the Pfizer deal will
help expand the choice of affordable contraceptives
Read more at: http://www.pharmatimes.com/Article/14-11-13/Pfizer_Gates_Foundation_to_expand_birth_control_access.aspx#ixzz3JIwfqmLy
Labels:
Big Pharma,
cognitive,
deconstruction,
future foresight,
numbers,
paradigm,
politics,
shit of the day
Sunday, November 16, 2014
We win, you lose - sign here!

Labels:
chaos,
cognitive,
deconstruction,
failure,
nice idea,
politics,
shit of the day
Friday, November 14, 2014
Wednesday, November 12, 2014
Russian Ruble. The falling.
Labels:
chaos,
deconstruction,
failure,
future foresight,
nice idea,
paradigm,
politics,
real science,
shit of the day
Tuesday, November 11, 2014
Too simple to be true!
Labels:
bubble,
deconstruction,
failure,
future foresight,
music of the week,
nice idea,
politics,
real science,
shit of the day
Dendreon - THE disaster!
http://finance.yahoo.com/news/biotech-dendreon-files-bankruptcy-protection-130212961.html
Shares fall as much as 74.5 pct!
What's wrong with vaccine???
Shares fall as much as 74.5 pct!
What's wrong with vaccine???
Friday, November 7, 2014
Thursday, November 6, 2014
Propaganda
Labels:
Big Pharma,
bubble,
cognitive,
deconstruction,
evidence based medicine,
failure,
future foresight,
paradigm,
real science,
shit of the day,
video
Sunday, November 2, 2014
The expert...
Labels:
chaos,
cognitive,
deconstruction,
depression,
evidence based medicine,
failure,
paradigm,
real science,
video
Friday, October 31, 2014
The biomedical revolution has begun!
Is it serious?:
These developments were being led by personalised medicine and pan-omics
(genomics, epigenomics, metabolomics, proteomics and exposomics), which would
radically change the way we understand diseases, he said. “We will unpick the
omics and patients will be put into a variety of categories based on whether the
drug will work and its toxicity.”
Thursday, October 30, 2014
Nanomadness?
So this nanoparticle platform we’re talking about essentially does the following: You take a capsule chock full of the nanoparticles, and they absorb into your body and into your bloodstream. These nanoparticles are two thousand times smaller than a single red blood cell. They’re tiny. They’re so little that they can pass through parts of your body, they go through the blood, they go through your lymph system, they just walk around. They’re essentially very benign particles—-there’s already lots of FDA approved nanoparticles for imaging and stuff like that, because they’re simply made out of an iron oxide core, like you take in a One-A-Day Plus Iron pill. And they’re decorated with proteins and amino acids and DNA to make them bind to certain things.
Full story: https://medium.com/backchannel/were-hoping-to-build-the-tricorder-12e1822e5e6a
Labels:
another magic cure,
Death Valley,
deconstruction,
future foresight,
nanotechnology,
paradigm,
quantum,
real science
Wednesday, October 29, 2014
“We are aggressively looking at all alternatives.” No balls - no babies!
The U.S. attempt to block companies from leaving the country for tax reasons wouldn’t stop Pfizer Inc. (PFE) from moving America’s biggest drugmaker overseas if it finds an attractive deal, Chief Executive Officer Ian Read said.
“If we believe the value is still there and we believe, under our interpretation of these rules, there is still value, I see no reason why we wouldn’t be able to do an inversion,” Read said yesterday in a telephone interview.
The ideal transaction has all three components, Read said, though U.S. Treasury Department rules announced last month could lower the value Pfizer assigns to the tax advantages. A tax inversion isn’t required for a deal, Read has said.
“Certainly I feel a sense of urgency on utilizing our balance sheet and our capital to do deals that are incremental, add incremental value and certainly add revenue growth in the innovative space,” Read said on a conference call with analysts today. “We are aggressively looking at all alternatives.”
Labels:
another magic cure,
Big Pharma,
chaos,
cognitive,
deconstruction,
future foresight,
masterpiece of the day,
nice idea,
numbers,
paradigm,
quote of the day,
real science
Monday, October 27, 2014

Labels:
another magic cure,
cognitive,
deconstruction,
evidence based medicine,
nice idea,
paradigm,
real science
The top 10 most expensive drugs of 2013. Future goes orphan!
Among the 20 most expensive drugs, only hep C fighter Sovaldi, Roche's breast cancer drug Kadcyla and Bristol-Myers Squibb's ($BMY) colon cancer drug Erbitux are not orphans, drugs approved under a special program to motivate companies to develop treatments for conditions that usually have patient populations of fewer than 200,000.
http://www.fiercepharma.com/special-reports/top-10-most-expensive-drugs-2013
Labels:
Big Pharma,
bubble,
deconstruction,
failure,
future foresight,
numbers,
paradigm,
real science
Side effects? Who cares?
■Iclusig, a $9,200-a-month leukemia drug that also has not been shown to lengthen lives. The FDA required a black box warning because clots formed in at least 27% of patients leading to possible heart attacks and strokes, potentially fatal heart failure and potentially fatal liver failure.
■Ixempra, a $7,600-a-month drug that improved the scans of advanced breast cancer patients for about a month and a half, but carried a warning for severe liver toxicity and a type of potentially fatal infection.
Critics say using surrogate measures to determine if a drug should be approved can backfire. Surrogates can mask complications that work against survival or quality of life, said Richard Deyo, a professor of evidence-based medicine at Oregon Health and Science University.
"Maybe you live a month longer with a new drug but you are having horrible symptoms or horrible quality of life in the meantime," said Deyo, author of "Hope or Hype: The Obsession with Medical Advances and the High Cost of False Promises."
"Patients would want to know that."
And much more...
Saturday, October 25, 2014
Personalized cancer vaccines? WTF?
Cancer vaccine developers have seen their fair share of disappointments, and failures have spurred some companies to test their treatments in smaller patient subpopulations. Now, researchers at the University of Connecticut are narrowing their focus even further as they gear up to trial personalized cancer vaccines.
Using genome sequencing to identify the differences between protein sequences--called epitopes--in healthy versus cancerous tissue, they'll start up an initial clinical study for ovarian cancer patients once they nab FDA approval. The team will sequence DNA from the tumors of 15 to 20 women with ovarian cancer, using that information to make a personalized vaccine for each woman.
Basically it means that cancer vaccines do not work... Surprised? I am not...
Thursday, October 23, 2014
Because they are worth it!
The first step of the process will involve construction of an in vivo center for animal research, slated for completion in 2018. And Roche--the top R&D spender in the world with a 2013 budget of $10.3 billion--plans to clear away older buildings to make way for a new office tower as part of a wider building plan that will cost a total of $3.2 billion.
Labels:
Big Pharma,
bubble,
deconstruction,
macro economy,
nice idea,
numbers,
paradigm,
real science
Tuesday, October 21, 2014
Monday, October 20, 2014
Fake?

Labels:
chaos,
cognitive,
deconstruction,
failure,
masterpiece of the day,
politics,
real science,
shit of the day
Sunday, October 19, 2014
Saturday, October 18, 2014
Size does matters!
Labels:
another magic cure,
cognitive,
deconstruction,
evidence based medicine,
nice idea,
numbers,
real science
That's it!
Labels:
deconstruction,
failure,
real science,
shit of the day,
video
Wednesday, October 15, 2014
2014's most successful biotech IPOs
http://www.fiercebiotech.com/special-reports/2014s-most-successful-biotech-ipos
2014's most successful biotech IPOs
2014's most successful biotech IPOs
Labels:
Big Pharma,
bubble,
deconstruction,
music of the week,
nice idea,
numbers,
paradigm,
real science
The top 10 most expensive drugs of 2013
The top 10 most expensive drugs of 2013 http://www.fiercepharma.com/special-reports/top-10-most-expensive-drugs-2013
Labels:
Big Pharma,
bubble,
investment,
nice idea,
numbers,
real science
Tuesday, October 14, 2014
The fantasy: 'spray-on-skin' for leg ulcers... WTF?
The product, known as HP802-247, was viewed by some analysts as a key pipeline asset in the company's advanced wound management division and the Phase III failure is a setback for the healthcare group, which is a regular subject of takeover talk.
The unsuccessful North American trial, announced on Monday, is also something of a surprise, given the promise of earlier studies.
http://in.reuters.com/article/2014/10/13/us-smith-nephew-skin-idINKCN0I20EN20141013?feedType=RSS&feedName=health&utm_source=feedburner&utm_medium=feed&utm_campaign=Feed%253A+reuters%252FINhealth+%2528News+%252F+IN+%252F+Health%2529
Labels:
chaos,
Death Valley,
deconstruction,
failure,
real science,
shit of the day,
targeted failure
Subscribe to:
Comments (Atom)